Pharmacokinetics of cefuroxime in infants and neonates undergoing cardiac surgery
- PMID: 29761538
- PMCID: PMC6089814
- DOI: 10.1111/bcp.13632
Pharmacokinetics of cefuroxime in infants and neonates undergoing cardiac surgery
Abstract
Aims: Very little data exist regarding the effect of cardiopulmonary bypass (CPB) on cefuroxime (CXM) pharmacokinetics in children less than one year of age.
Methods: 50 mg kg-1 CXM i.v. after induction were followed by 75 mg kg-1 into the CPB circuit. In 42 patients undergoing cardiac surgery, 15-20 samples were obtained between 5 and 360 min after the first dose. Total CXM concentrations were measured by high-performance liquid chromatography and a pharmacokinetic/pharmacodynamic (PK/PD) modelling was performed.
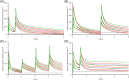
Results: Using a fixed protein binding of 15.6% for CXM, peak plasma concentrations of unbound CXM were 229 ± 52 μg ml-1 after the first bolus and 341 ± 86 μg ml-1 on CPB. Nadir concentrations before CPB were 69 ± 20 μg ml-1 and six hours later decreased to 41 ± 19 μg ml-1 with and 24 ± 14 μg ml-1 without CPB. A two-compartment model was fitted with the main covariates body weight, CPB and postmenstrual age (PMA). PK parameters were as follows: systemic clearance, 5.15 [95% CI 4.5-5.8] l h-1 ; central volume of distribution, 11.25 [9.41-13.09] l; intercompartmental clearance, 18.19 [14.79-21.58] l h-1 ; and peripheral volume, 17.07 [15.7-18.5] L. ƒT > MIC of 32 μg ml-1 for an 8-h time period was between 70 and 100% (2.5-10 kg BW). According to our simulation, 25 mg ml-1 CXM as a primary bolus and into the prime plus a 5 mg kg-1 h-1 infusion maintain CXM concentrations continuously above 32 μg ml-1 .
Conclusions: The routine dosing regimen provided was sufficient for prophylaxis, but continuous dosing can provide a higher percentage of ƒT > MIC.
Keywords: antibiotic prophylaxis; cardiac surgical procedures; cefuroxime; heart defects, congenital; pharmacokinetics.
© 2018 The British Pharmacological Society.
Figures
Comment in
-
Model antibiotic use to improve outcomes.Br J Clin Pharmacol. 2021 Mar;87(3):738-740. doi: 10.1111/bcp.14559. Epub 2020 Oct 19. Br J Clin Pharmacol. 2021. PMID: 33078437 No abstract available.
References
-
- Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al American Society of Health‐System P, Infectious Disease Society of A, Surgical Infection S, Society for Healthcare Epidemiology of A. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013; 70: 195–283. - PubMed
-
- Costello JM, Graham DA, Morrow DF, Morrow J, Potter‐Bynoe G, Sandora TJ, et al Risk factors for surgical site infection after cardiac surgery in children. Ann Thorac Surg 2010; 89: 1833–1841. - PubMed
-
- Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, et al Pharmacokinetics‐pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 2007; 44: 79–86. - PubMed
-
- The European Committee on Antimicrobial Susceptibility Testing . Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0. 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

