Influence of collagen-based integrin α1 and α2 mediated signaling on human mesenchymal stem cell osteogenesis in three dimensional contexts
- PMID: 29761640
- PMCID: PMC7147932
- DOI: 10.1002/jbm.a.36451
Influence of collagen-based integrin α1 and α2 mediated signaling on human mesenchymal stem cell osteogenesis in three dimensional contexts
Abstract
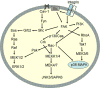
Collagen I interactions with integrins α1 and α2 are known to support human mesenchymal stem cell (hMSC) osteogenesis. Nonetheless, elucidating the relative impact of specific integrin interactions has proven challenging, in part due to the complexity of native collagen. In the present work, we employed two collagen-mimetic proteins-Scl2-2 and Scl2-3- to compare the osteogenic effects of integrin α1 versus α2 signaling. Scl2-2 and Scl2-3 were both derived from Scl2-1, a triple helical protein lacking known cell adhesion, cytokine binding, and matrix metalloproteinase sites. However, Scl2-2 and Scl2-3 were each engineered to display distinct collagen-based cell adhesion motifs: GFPGER (binding integrins α1 and α2 ) or GFPGEN (binding only integrin α1 ), respectively. hMSCs were cultured within poly(ethylene glycol) (PEG) hydrogels containing either Scl2-2 or Scl2-3 for 2 weeks. PEG-Scl2-2 gels were associated with increased hMSC osterix expression, osteopontin production, and calcium deposition relative to PEG-Scl2-3 gels. These data indicate that integrin α2 signaling may have an increased osteogenic effect relative to integrin α1 . Since p38 is activated by integrin α2 but not by integrin α1 , hMSCs were further cultured in PEG-Scl2-2 hydrogels in the presence of a p38 inhibitor. Results suggest that p38 activity may play a key role in collagen-supported hMSC osteogenesis. This knowledge can be used toward the rational design of scaffolds which intrinsically promote hMSC osteogenesis. © 2018 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 106A: 2594-2604, 2018.
Keywords: collagen-mimetic proteins; human mesenchymal stem cells; integrin α1; integrin α2; osteogenesis.
Copyright © 2018 Wiley Periodicals, Inc.
Figures


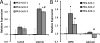

References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284(5411):143–147. - PubMed
-
- Donzelli E, Salvadè A, Mimo P, Viganò M, Morrone M, Papagna R, Carini F, Zaopo A, Miloso M, Baldoni M, et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Archives of Oral Biology. 2007;52(1):64–73. - PubMed
-
- Chan BP, Hui TY, Wong MY, Yip KHK, Chan GCF. Mesenchymal Stem Cell–Encapsulated Collagen Microspheres for Bone Tissue Engineering. Tissue Engineering Part C: Methods. 2009;16(2):225–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

