The Role of Structural Enthalpy in Spherical Nucleic Acid Hybridization
- PMID: 29762017
- PMCID: PMC6001361
- DOI: 10.1021/jacs.8b03459
The Role of Structural Enthalpy in Spherical Nucleic Acid Hybridization
Abstract
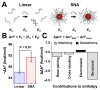
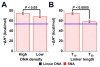
DNA hybridization onto DNA-functionalized nanoparticle surfaces (e.g., in the form of a spherical nucleic acid (SNA)) is known to be enhanced relative to hybridization free in solution. Surprisingly, via isothermal titration calorimetry, we reveal that this enhancement is enthalpically, as opposed to entropically, dominated by ∼20 kcal/mol. Coarse-grained molecular dynamics simulations suggest that the observed enthalpic enhancement results from structurally confining the DNA on the nanoparticle surface and preventing it from adopting enthalpically unfavorable conformations like those observed in the solution case. The idea that structural confinement leads to the formation of energetically more stable duplexes is evaluated by decreasing the degree of confinement a duplex experiences on the nanoparticle surface. Both experiment and simulation confirm that when the surface-bound duplex is less confined, i.e., at lower DNA surface density or at greater distance from the nanoparticle surface, its enthalpy of formation approaches the less favorable enthalpy of duplex formation for the linear strand in solution. This work provides insight into one of the most important and enabling properties of SNAs and will inform the design of materials that rely on the thermodynamics of hybridization onto DNA-functionalized surfaces, including diagnostic probes and therapeutic agents.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures


Similar articles
-
What controls the hybridization thermodynamics of spherical nucleic acids?J Am Chem Soc. 2015 Mar 18;137(10):3486-9. doi: 10.1021/jacs.5b00670. Epub 2015 Mar 4. J Am Chem Soc. 2015. PMID: 25738968 Free PMC article.
-
Effects for the incorporation of five-atom thioacetamido nucleic acid (TANA) backbone on hybridization thermodynamics and kinetics of DNA duplexes.J Phys Chem B. 2009 Mar 5;113(9):2944-51. doi: 10.1021/jp808747g. J Phys Chem B. 2009. PMID: 19708120
-
Significance of DNA bond strength in programmable nanoparticle thermodynamics and dynamics.Soft Matter. 2018 Apr 4;14(14):2665-2670. doi: 10.1039/C7SM02456H. Soft Matter. 2018. PMID: 29561032
-
Freezing Functional Nucleic Acids: From Molecular Reactions to Surface Immobilization.Chembiochem. 2024 Dec 2;25(23):e202400416. doi: 10.1002/cbic.202400416. Epub 2024 Sep 3. Chembiochem. 2024. PMID: 38979890 Review.
-
Strategies for optimizing DNA hybridization on surfaces.Anal Biochem. 2014 Jan 1;444:41-6. doi: 10.1016/j.ab.2013.09.032. Epub 2013 Oct 9. Anal Biochem. 2014. PMID: 24121011 Review.
Cited by
-
Spherical Nucleic Acids: Integrating Nanotechnology Concepts into General Chemistry Curricula.J Chem Educ. 2021 Oct 12;98(10):3090-3099. doi: 10.1021/acs.jchemed.1c00441. Epub 2021 Sep 8. J Chem Educ. 2021. PMID: 35250048 Free PMC article.
-
Poly-adenine-mediated spherical nucleic acids for interfacial recognition of kanamycin.Mikrochim Acta. 2022 Mar 22;189(4):151. doi: 10.1007/s00604-022-05235-3. Mikrochim Acta. 2022. PMID: 35316405
-
Engineering DNA-Functionalized Nanostructures to Bind Nucleic Acid Targets Heteromultivalently with Enhanced Avidity.J Am Chem Soc. 2020 May 27;142(21):9653-9660. doi: 10.1021/jacs.0c01568. Epub 2020 May 14. J Am Chem Soc. 2020. PMID: 32338896 Free PMC article.
-
Distinct structural interactions of polyadenine and polythymine on gold nanoparticles: from single strands to duplexes.Chem Sci. 2025 Jul 31;16(35):15947-15954. doi: 10.1039/d5sc04459f. eCollection 2025 Sep 10. Chem Sci. 2025. PMID: 40787213 Free PMC article.
-
The effector mechanism of siRNA spherical nucleic acids.Proc Natl Acad Sci U S A. 2020 Jan 21;117(3):1312-1320. doi: 10.1073/pnas.1915907117. Epub 2020 Jan 3. Proc Natl Acad Sci U S A. 2020. PMID: 31900365 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

