L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis
- PMID: 29762873
- PMCID: PMC6494563
- DOI: 10.1002/14651858.CD012410.pub2
L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis
Abstract
Background: Hepatic encephalopathy is a common complication of cirrhosis and has high associated morbidity and mortality. The condition is classified as overt if it is clinically apparent or minimal if only evident though psychometric testing. The exact pathogenesis of this syndrome is unknown although ammonia is thought to play a key role. L-ornithine L-aspartate has ammonia-lowering properties and may, therefore, benefit people with cirrhosis and hepatic encephalopathy.
Objectives: To evaluate the beneficial and harmful effects of L-ornithine L-aspartate versus placebo, no intervention, or other active interventions in people with cirrhosis and hepatic encephalopathy.
Search methods: We undertook electronic searches of The Cochrane Hepato-Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, LILACS and Science Citation Index Expanded to December 2017 and manual searches of meetings and conference proceedings; checks of bibliographies; and corresponded with investigators and pharmaceutical companies.
Selection criteria: We included randomised clinical trials, irrespective of publication status, language, or blinding. We included participants with cirrhosis who had minimal or overt hepatic encephalopathy or who were at risk for developing hepatic encephalopathy. We compared: L-ornithine L-aspartate versus placebo or no intervention; and L-ornithine L-aspartate versus other active agents such as non-absorbable disaccharides, antibiotics, probiotics, or branched-chain amino acids.
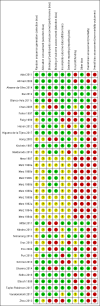
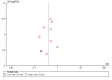
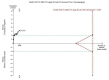
Data collection and analysis: Two review authors, working independently, retrieved data from published reports and correspondence with investigators and pharmaceutical companies. The primary outcomes were mortality, hepatic encephalopathy, and serious adverse events. We undertook meta-analyses and presented the results as risk ratios (RR) and mean differences (MD) with 95% confidence intervals (CI). We assessed bias control using the Cochrane Hepato-Biliary Group domains; we evaluated the risk of publication bias and other small trial effects in regression analyses; conducted subgroup and sensitivity analyses; and performed Trial Sequential Analyses. We determined the quality of the evidence using GRADE.
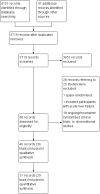
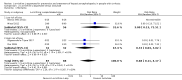
Main results: We identified 36 randomised clinical trials, involving at least 2377 registered participants, which fulfilled our inclusion criteria including 10 unpublished randomised clinical trials. However, we were only able to access outcome data from 29 trials involving 1891 participants. Five of the included trials assessed prevention, while 31 trials assessed treatment. Five trials were at low risk of bias in the overall assessment of mortality; one trial was at low risk of bias in the assessment of the remaining outcomes.L-ornithine L-aspartate had a beneficial effect on mortality compared with placebo or no intervention when including all trials (RR 0.42, 95% CI 0.24 to 0.72; I2 = 0%; 19 trials; 1489 participants; very low quality evidence), but not when the analysis was restricted to the trials at low risk of bias (RR 0.47, 95% CI 0.06 to 3.58; 4 trials; 244 participants). It had a beneficial effect on hepatic encephalopathy compared with placebo or no intervention when including all trials (RR 0.70, 95% CI 0.59 to 0.83; 22 trials; 1375 participants; I2 = 62%; very low quality evidence), but not in the one trial at low risk of bias (RR 0.96, 95% CI 0.85 to 1.07; 63 participants). The analysis of serious adverse events showed a potential benefit of L-ornithine L-aspartate when including all randomised clinical trials (RR 0.63, 95% CI 0.45 to 0.90; 1 trial; 1489 participants; I2 = 0%; very low quality evidence), but not in the one trial at low risk of bias for this outcome (RR 0.83, 95% CI 0.15 to 4.65; 63 participants). The Trial Sequential Analyses of mortality, hepatic encephalopathy, and serious adverse events found insufficient evidence to support or refute beneficial effects. Subgroup analyses showed no difference in outcomes in the trials evaluating evaluating the prevention or treatment of either overt or minimal hepatic encephalopathy or trials evaluating oral versus intravenous administration We were unable to undertake a meta-analysis of the three trials involving 288 participants evaluating health-related quality of life. Overall, we found no difference between L-ornithine L-aspartate and placebo or no intervention in non-serious adverse events (RR 1.15, 95% CI 0.75 to 1.77; 14 trials; 1076 participants; I2 = 40%). In comparison with lactulose, L-ornithine L-aspartate had no effect on mortality (RR 0.68, 95% CI 0.11 to 4.17; 4 trials; 175 participants; I2 = 0%); hepatic encephalopathy (RR 1.13, 95% CI 0.81 to 1.57); serious adverse events (RR 0.69, 95% CI 0.22 to 2.11); or non-serious adverse events (RR 0.05, 95% CI 0.01 to 0.18). In comparison with probiotics, L-ornithine L-aspartate had no effect on mortality (RR 1.01, 95% CI 0.11 to 9.51); serious adverse events (RR 1.07, 95% CI 0.23 to 4.88); or changes in blood ammonia concentrations from baseline (RR -2.30 95% CI -6.08 to 1.48), but it had a possible beneficial effect on hepatic encephalopathy (RR 0.71, 95% CI 0.56 to 0.90). Finally, in comparison with rifaximin, L-ornithine L-aspartate had no effect on mortality (RR 0.33, 95% CI 0.04 to 3.03; 2 trials; 105 participants); hepatic encephalopathy (RR 1.06, 95% CI 0.57 to 1.96); serious adverse events (RR 0.32, 95% CI 0.01 to 7.42), or non-serious adverse events (RR 0.32, 95% CI 0.01 to 7.42).
Authors' conclusions: The results of this review suggest a possible beneficial effect of L-ornithine L-aspartate on mortality, hepatic encephalopathy, and serious adverse events in comparisons with placebo or no-intervention, but, because the quality of the evidence is very low, we are very uncertain about these findings. There was very low quality evidence of a possible beneficial effect of L-ornithine L-aspartate on hepatic encephalopathy, when compared with probiotics, but no other benefits were demonstrated in comparison with other active agents. Additional access to data from completed, but unpublished trials, and new randomised placebo-controlled, double-blind clinical trials are needed.
Conflict of interest statement
ETG: nothing to declare. CSS: nothing to declare. SS: nothing to declare. HV: nothing to declare. LLG: Abbvie, Merck, Norgine (investigator in trials), Novo Nordisk (travel expenses), Norgine (teaching), and Alexion (funding for research). MYM: nothing to declare.
Figures






















Update of
- doi: 10.1002/14651858.CD012410
References
References to studies included in this review
Abid 2011 {published data only}
-
- Abid S, Jafri W, Mumtaz K, Islam M, Abbas Z, Shah HA, et al. Efficacy of L‐ornithine‐L‐aspartate as an adjuvant therapy in cirrhotic patients with hepatic encephalopathy. Journal of the College of Physicians and Surgeons Pakistan 2011;21:666‐71. [PUBMED: 22078345] - PubMed
-
- Abid S, Jafri W, Mumtaz K, Islam M, Abbas Z, Shah HA, et al. Efficacy of infusion of L‐ornithine L‐aspartate in cirrhotic patients with portosystemic encephalopathy: a placebo controlled study [abstract]. Journal of Hepatology 2005;42(Suppl 2):84.
Ahmad 2008 {published data only}
-
- Ahmad I, Khan AA, Alam A, Dilshad A, Butt AK, Shafqat F, et al. L‐ornithine‐L‐aspartate infusion efficacy in hepatic encephalopathy. Journal of the College of Physicians and Surgeons Pakistan 2008;18:684‐7. [PUBMED: 18983791] - PubMed
Alvares‐da‐Silva 2014 {published data only}
-
- Alvares‐da‐Silva MR, Araujo A, Vicenzi JR, Oliveira FB, Silva GV, Schacher FC, et al. Oral L‐ornithine L‐aspartate (LOLA) in cirrhotic patients with minimal hepatic encephalopathy (MHE): final results of a randomized double‐blind placebo‐controlled trial (PORTO ALEGRE STUDY) [abstract]. Hepatology (Baltimore, Md.) 2011;54(Suppl 1):1249A.
-
- Alvares‐da‐Silva MR, Araujo A, Vicenzi JR, Silva GV, Oliveira FB, Schacher F, et al. Oral L‐ornithine‐L‐aspartate in minimal hepatic encephalopathy: a randomized, double‐blind, placebo‐controlled trial. Hepatology Research 2014;44:956‐63. [NCT00896831; PUBMED: 24033861] - PubMed
-
- Silva GD, Alvares‐Da‐Silva M, Araujo AD, Vicenzi J, Magnus A, Bazzanella F, et al. Therapeutic efficacy of oral L‐ornithine‐L‐aspartate on minimal encephalopathy (PORTOALEGRE). www.lume.ufrgs.br/bitstream/handle/10183/38555/000793877.pdf?sequence=1 2014 (accessed 3 October 2017).
Bai 2014 {published data only}
-
- Bai M, He C, Yin Z, Niu J, Wang Z, Qi X, et al. Randomised clinical trial: L‐ornithine‐l‐aspartate reduces significantly the increase of venous ammonia concentration after TIPSS. Alimentary Pharmacology & Therapeutics 2014; Vol. 40:63‐71. [DOI: ; CN‐00992348] - PubMed
Blanco Vela 2011c {published and unpublished data}
-
- Blanco Vela CI, Bosques Padilla FJ. L‐ornithine L‐aspartate is as effective as nonabsorbable disaccharides in the management of acute hepatic encephalopathy [abstract]. Hepatology (Baltimore, Md.) 2011;54(Suppl):1899.
Chen 2005 {published data only}
-
- Chen MF, Li RC, Chen CH, Gao XC. Therapeutic effect of L‐ornithine‐L‐aspartate on liver cirrhosis complicated by hepatic encephalopathy. Di Yi Jun Yi Da Xue Xue Bao 2005;25:718‐22. - PubMed
Feher 1997 {published data only}
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
-
- Feher J, Lang I, Gogl A, Varga L, Varga L, Tompos G, et al. Effect of ornithine‐aspartate infusion on elevated serum ammonia concentration in cirrhotic patients ‐ results of a randomized, placebo‐controlled double‐blind multicentre trial. Medical Science Monitor 1997;3:669‐73.
Fleig 1999 {published and unpublished data}
-
- Fleig WE, Kircheis G, Spengler U, Zeuzem S, Görtelmeyer R, the German‐Austrian‐Swiss Multicenter Study Group. Placebo‐controlled, double‐blind evaluation of L‐ornithine–L‐aspartate (LOLA) granules in patients with cirrhosis and subclinical (SHE) or mild overt hepatic encephalopathy (HE) [abstract]. Journal of Hepatology 1999;30(Suppl):65.
-
- Kircheis G, Fleig WE, Görtelmeyer R, Grafe S, Häussinger D. Assessment of low‐grade hepatic encephalopathy: a critical analysis. Journal of Hepatology 2007;47:642‐50. [PUBMED: 17869373] - PubMed
Hasan 2012 {published data only}
-
- Hasan I, Iskandar M, Budihusodo U. Effect of L‐ornithine‐L‐aspartate on liver cirrhosis patients with low‐grade hepatic encephalopathy [abstract]. Hepatology International 2012; Vol. 6:295. [DOI: ; CN‐01005005]
Higuera‐de la Tijera 2017 {published and unpublished data}
-
- Higuera‐de la Tijera F. A randomized, double‐blind, clinical trial comparing lactulose, L‐ornithine L‐aspartate, or rifaximin, versus placebo, as primary prophylaxis of hepatic encephalopathy in cirrhotic patients with variceal bleeding. Journal of Hepatology 2017;66(Suppl 1):S49. [NCT02158182]
Hong 2003 {published data only}
-
- Hong Y, Yang Z, Wang Y. Clinical control study of L‐ornithine‐L‐aspartate in the treatment of subclinical hepatic encephalopathy. West China Medical Journal 2003;4:508‐10.
Kircheis 1997 {published data only}
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
-
- Kircheis G, Nilius R, Held C, Berndt H, Buchner M, Gortelmeyer R, et al. Therapeutic efficacy of L‐ornithine‐L‐aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo‐controlled, double‐blind study. Hepatology (Baltimore, Md.) 1997;25:1351‐60. [PUBMED: 9185752] - PubMed
-
- Kircheis G, Nilius R, Held C, Berndt H, Buchner M, Gortelmeyer R, et al. Therapeutic efficacy of L‐ornithine‐L‐aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo‐controlled, double‐blind study [abstract]. Hepatology Research 1997;8:221. - PubMed
Maldonado 2010 {published data only}
-
- Maldonado HJ, Torres JOG, Vazquez‐Elizondo G, Sandoval MGC, Garza‐Gonzalez E, Bosques FJ. Clinical efficacy of L‐ornithine L‐aspartate in the prevention of altered oral glutamine challenge in patients with minimal hepatic encephalopathy: a pilot study [abstract]. Gastroenterology 2010;138(Suppl 1):S818.
Merz 1987 {unpublished data only}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
Merz 1988a {unpublished data only}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
Merz 1988b {published and unpublished data}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
-
- Delcker AM, Jalan R, Schumacher M, Comes, G. L‐ornithine‐L‐aspartine vs placebo in the treatment of hepatitis encephalopathy: meta‐analysis of randomised placebo‐controlled trials using individual data (MRZ 90004‐8806). Hepatology (Baltimore, Md.) 2000;32(4 Part 2):310A.
Merz 1988c {published and unpublished data}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
-
- Delcker AM, Jalan R, Schumacher M, Comes G. L‐ornithine‐L‐aspartine vs placebo in the treatment of hepatitis encephalopathy: meta‐analysis of randomised placebo‐controlled trials using individual data (MRZ 90004‐8807). Hepatology (Baltimore, Md.) 2000;32(4 Part 2):310A.
Merz 1988d {unpublished data only}
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
Merz 1989a {published and unpublished data}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
-
- Delcker AM, Jalan R, Schumacher M, Comes, G. L‐ornithine‐L‐aspartine vs placebo in the treatment of hepatitis encephalopathy: meta‐analysis of randomised placebo‐controlled trials using individual data (MRZ 90004‐8901). Hepatology (Baltimore, Md.) 2000;32(4 Part 2):310A.
Merz 1989b {unpublished data only}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
Merz 1992a {unpublished data only}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
Merz 1994a {unpublished data only}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
Merz 1994b {unpublished data only}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
Mittal 2011 {published data only}
-
- Mittal VV, Sharma BC, Sharma P, Sarin SK. A randomized controlled trial comparing lactulose, probiotics, and L‐ornithine L‐aspartate in treatment of minimal hepatic encephalopathy. European Journal of Gastroenterology and Hepatology 2011;23:725‐32. [PUBMED: 21646910] - PubMed
-
- Mittal VV, Sharma P, Sharma B, Sarin SK. Treatment of minimal hepatic encephalopathy: a randomized controlled trial comparing lactulose, probiotics & L‐ornithine L‐aspartate with placebo [abstract]. Hepatology (Baltimore, Md.) 2009; Vol. 50, issue Suppl 2:471A. [CN‐00739663] - PubMed
Ndraha 2011 {published data only}
-
- Ndraha S, Hasan I, Simadibrata M. The effect of L‐ornithine L‐aspartate and branch chain amino acids on encephalopathy and nutritional status in liver cirrhosis with malnutrition. Acta Medica Indonesiana 2011;43:18‐22. [PUBMED: 21339541] - PubMed
Nimanong 2010 {published data only}
-
- Nimanong S, Siwaporn C, Sansak I, Tanwandee T, Charatcharoenwitthaya P, Chotiyaputta W, et al. Oral L‐ornithine‐L‐aspartate (LOLA) for patients with overt hepatic encephalopathy treated with lactulose; a randomized, double‐blinded, placebo‐controlled trial [abstract]. Hepatology International 2010;4:35.
-
- Nimanong S, Siwaporn C, Sansak I, Tanwandee T, Charatcharoenwitthaya P, Chotiyaputta W, et al. Oral L‐ornithine‐L‐aspartate (LOLA) for patients with overt hepatic encephalopathy treated with lactulose; a randomized, double‐blinded, placebo‐controlled trial [abstract]. Journal of Hepatology 2010;52(Suppl 1):S78.
Oruc 2010 {published data only}
-
- Oruc N, Ozturk A, Onal IK, Unal NG, Akdogan M, Yurdaydin C, et al. Therapeutic efficacy of L‐ornithine‐L‐aspartate infusions in patents with cirrhosis and hepatic encephalopathy [abstract]. Journal of Hepatology 2010;52(Suppl 1):S78‐9.
Poo 2006 {published data only}
-
- Poo JL, Gongora J, Sanchez‐Avila F, Aguilar‐Castillo S, Garcia‐Ramos G, Fernandez‐Zertuche M, et al. Efficacy of oral L‐ornithine‐L‐aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose‐controlled study. Annals of Hepatology 2006;5:281‐8. [PUBMED: 17151582] - PubMed
-
- Poo JL, Gongora J, Sanchez‐Avila JF, Aguilar‐Castillo S, Garcia Ramos G, Fernandez‐Zertuche M, et al. Efficacy and safety of L‐ornithine L‐aspartate (LOLA) administration. Open label randomized controlled trial versus lactulose in cirrhotic patients with hyperammoniemic hepatic encephalopathy [abstract]. Journal of Hepatology 2007;46(Suppl 1):S36. - PubMed
Puri 2010 {published data only}
-
- Puri P, Dhingra A, Nandi B, Tiwari A, Grover N, Srivastav S, et al. Treatment of minimal hepatic encephalopathy: a randomized control trial comparing L ornithine L aspartate with placebo [abstract]. Indian Journal of Gastroenterology 2010;29:65.
Schmid 2010 {published data only}
-
- Schmid M, König M, Ferenci P, Gangl A, Peck‐Radosavljevic M. Prospective randomized pilot trial of I.V. L‐ornithine L‐aspartate vs. placebo on postural control in patients with cirrhosis [abstract]. Journal of Hepatology 2008;48(Suppl 2):S123.
-
- Schmid M, Peck‐Radosavljevic M, König F, Mittermaier C, Gangl A, Ferenci P. A double‐blind, randomized, placebo‐controlled trial of intravenous L‐ornithine‐L‐aspartate on postural control in patients with cirrhosis. Liver International 2010;30:574‐82. [DOI: 10.1111/j.1478-3231.2010.02213.x; CN‐00753299] - DOI - PubMed
Sharma 2014 {published data only}
Sidhu 2018 {published and unpublished data}
-
- NCT01722578. L‐ornithine L‐aspartate in overt hepatic encephalopathy (HEAL) [Efficacy of Intravenous 'L‐ornithine L‐aspartate' in reversal of overt acute hepatic encephalopathy in patients with liver cirrhosis: a prospective, randomized, double‐blind, placebo controlled trial]. clinicaltrials.gov/ct2/show/NCT01722578 2017.
Stauch 1998 {published data only}
-
- Stauch S, Kircheis G, Adler G, Beckh K, Ditschuneit H, Gortelmeyer R, et al. Oral L‐ornithine‐L‐aspartate therapy of chronic hepatic encephalopathy: results of a placebo‐controlled double‐blind study. Journal of Hepatology 1998;28:856‐64. [PUBMED: 9625322] - PubMed
Taylor‐Robinson 2017 {published and unpublished data}
-
- Pasha Y, Leech R, Violante IR, Cook N, Crossey MME, Taylor‐Robinson SD. Brain muscle axis during treatment of hepatic encephalopathy with L‐ornithine L‐aspartate. Journal of Clinical and Experimental Hepatology 2017;7(Suppl 1):S5‐6.
Varakanahalli 2017 {published data only}
-
- Varakanahalli S, Sharma BC, Dahale AS. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: a double blind randomized controlled trial of L‐ornithine L‐aspartate vs placebo. Journal of Clinical and Experimental Hepatology 2017;7(Suppl 2):S60‐1. [DOI: ] - PubMed
Zhou 2013 {published data only}
-
- Zhou ZW, Zhong XN, Zhou BY, Xiang JF, Wang RH, Yi J. Ornithine aspartate and naloxone combined therapy for hepatic encephalopathy affects cognitive function, prognosis, and neuropeptide levels. Chinese Journal of Hepatology 2013;21:385‐8. - PubMed
References to studies excluded from this review
Abdo‐Francis 2010 {published data only}
-
- Abdo‐Francis JM, Pérez‐Hernández JL, Hinojosa‐Ruiz A, Hernández‐Vásquez JR. Decreased hospital stay with the use of L‐ornithine L‐ aspartate (LOLA) in patients with hepatic encephalopathy [Disminución de la estancia hospitalaria conel uso de L‐ornitina L‐aspartato (LOLA) enpacientes con encefalopatía hepática]. Revista de Gastroenterología de México 2010;2:135‐41. [PUBMED: 20615780] - PubMed
Acharya 2009 {published data only}
Aidrus 2015 {published data only}
-
- Aidrus F, Razzaque S, Siddiqui A, Kumar A, Ghauri MI. Therapeutic efficacy of L‐ornithine L‐aspartate in patients with hepatic encephalopathy. Pakistan Journal of Neurological Sciences 2015;10:37‐41.
Badea 2015 {published data only}
-
- Badea M, Gavrilescu O, Dranga M, Mihai C, Prelipcean CC. Lactulose alone versus lactulose and L‐ornithine L‐aspartate for the prophylaxis of hepatic encephalopathy in acute variceal bleeding: a prospective case control study. Falk Symposium 199 2015:18.
Delcker 2002 {published data only}
-
- Delcker A, Turowski B, Mihm U, Raab P, Rusch O, Pilatus U, et al. Proton MR spectroscopy of neurometabolites in hepatic encephalopathy during L‐ornithine‐L‐aspartate treatment ‐ results of a pilot study. Metabolic Brain Disease 2002;17:103‐11. [PUBMED: 12083335] - PubMed
Grover 2017 {published and unpublished data}
-
- Grover VP, McPhail MJ, Wylezinska M, Crossey MM, Fitzpatrick JA, Southern L, et al. A longitudinal study of patients with cirrhosis treated with L‐ornithine L‐aspartate, examined with magnetization transfer, diffusion‐weighted imaging and magnetic resonance spectroscopy. Metabolic Brain Disease 2017; Vol. 32:77‐86. - PMC - PubMed
Lim 2010 {published data only}
-
- Lim TH, Gane E, Orr D. Oral L‐ornithine‐L‐aspartate is safe and effective in patients with intractable hepatic encephalopathy ‐ the NZ liver transplant unit experience. Hepatology International 2010;4:258.
McPhail 2013 {published data only}
Merz 1988e {unpublished data only}
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy (MRZ 90004‐8701). International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
Merz 1991 {unpublished data only}
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
Merz 1992b {unpublished data only}
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report: MRZ 90004‐9902 2000.
Müting 1980 {published data only}
-
- Müting D, Reikowski J. Controlled study of the effect of an orally administered ammonia lowering amino acid (ornithine‐aspartate) on liver and pancreas function in liver cirrhosis patients. Therapiewoche 1980;30:5590‐6.
-
- Reikowski J. Controlled study evaluating the effect of high dose oral ornithineaspartate on hepatic encephalopathy in patients with cirrhosis with or without surgical shunts [Kontrollierte Studie über die Wirkung von hohen Dosen Ornithinaspartate per os auf die Leberentgiftung bei Lebercirrhosen mit und ohne Shuntoperation]. Aminosäuren‐ und Ammoniakstoffwechsel bei Leberinsuffizienz. Grundlagen und therapeutische Folgerungen. Baden‐Baden, Germany: Gerhardt Witzstrock, 1982.
Ndhara 2010 {published data only}
-
- Ndraha S, Hasan I, Simadibrata M. The efficacy of L‐ornithine L‐aspartate granules and normal protein diet in minimal hepatic encephalopathy with malnutrition. Indonesian Journal of Gastroenterology, Hepatology, and Digestive Endoscopy 2010;11:11‐4.
Ong 2011 {published data only}
-
- Ong JP, Oehler G, Kruger‐Jansen C, Lambert‐Baumann J, Younossi ZM. Oral L‐ornithine‐L‐aspartate improves health‐related quality of life in cirrhotic patients with hepatic encephalopathy: an open‐label, prospective, multicentre observational study. Clinical Drug Investigation 2011;31:213‐20. [PUBMED: 21208014] - PubMed
Popa 2015 {published data only}
-
- Popa I, Badea M, Gavrilescu O, Dranga M, Chiosa AM, Didita A, et al. Normix alone vs. normix + L‐ornithine L‐aspartate for the treatment of patients with minimal hepatic encephalopathy. Falk Symposium 199 2015:69.
Rees 2000 {published data only}
-
- Rees CJ, Oppong K, Al‐Mardini H, Hudson M, Rose J, Record CO. The effect of L‐ornithine L‐aspartate on TIPS patients undergoing glutamine challenge?. In: Record C, Al‐Mardini H editor(s). Advances in Hepatic Encephalopathy & Metabolism in Liver Disease: Proceedings of the 9th International Symposium on Ammonia. Vol. 64, Newcastle upon Tyne (UK): Ipswich Book Company Ltd, 1997:459‐65.
Reikowski 1982 {published data only}
-
- Reikowski J, Müting D. Controlled study evaluating the effect of high dose ornithin‐aspartate on hepatic encephalopathy in cirrhosis with or without surgical shunts [Kontrollierte Studie von die Wirkung von hohen Dosen ornithinaspartat auf die Leberentgiftung bei Leberzirrhosen mit oder ohne Shuntoperation]. In: Holm E editor(s). Aminosäuren‐ und Ammoniakstoffwechsel bei Leberinsuffizienz. Grundlagen und therapeutische Folgerungen. Baden‐Baden (Germany): Witzstrock, 1982.
Staedt 1993 {published data only}
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report MRZ 90004‐8603.
-
- Staedt U, Leweling H, Gladisch R, Kortsik C, Hagmuller E, Holm E. Effects of ornithine aspartate on plasma ammonia and plasma amino acids in patients with cirrhosis. A double‐blind, randomized study using a four‐fold crossover design. Journal of Hepatology 1993;19:424‐30. [PUBMED: 8151104] - PubMed
Tenda 2012 {published data only}
-
- Tenda ED. Effect of BCAA and LOLA combination as late evening snacks on nutritional status and minimal hepatic encephalopathy in cirrhosis [abstract]. Hepatology Research 2013;7:2185.
-
- Tenda ED, Hasan I, Sanityoso A, Setiati S. The effect of branched chain amino acids and L‐ornithine L‐aspartate combination as the late evening snacks on nutritional status and minimal hepatic encephalopathy in liver cirrhosis. Indonesian Journal of Gastroenterology, Hepatology and Digestive Endoscopy 2012;13:151‐6.
Tiller 2016 {published data only}
-
- Tiller M, Bender M, Schepp W, Gundling F. Motor deficit of hand and arm movements in manifest hepatic encephalopathy before and after therapy with L‐ornithin‐L‐aspartat [abstract]. Gastroenterology 2016;150(Suppl 1):S1118.
Additional references
Atluri 2011
Bai 2013
-
- Bai M, Yang Z, Qi X, Fan D, Han G. L‐ornithine‐L‐aspartate for hepatic encephalopathy in patients with cirrhosis: a meta‐analysis of randomized controlled trials. Journal of Gastroenterology and Hepatology 2013;28:783‐92. [PUBMED: 23425108] - PubMed
Bajaj 2008
-
- Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology 2008;135:1591‐600. [PUBMED: 18723018] - PubMed
Bajaj 2009
-
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology (Baltimore, Md.) 2009;50:2014‐21. [PUBMED: 19787808] - PubMed
Bajaj 2010
Bajaj 2011a
-
- Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, et al. Review article: the design of clinical trials in hepatic encephalopathy ‐ an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Alimentary Pharmacology & Therapeutics 2011;33:739‐47. [PUBMED: 21306407] - PMC - PubMed
Bajaj 2011b
Bass 2010
-
- Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. New England Journal of Medicine 2010;362:1071‐81. [PUBMED: 20335583] - PubMed
Blanco Vela 2011a
-
- Blanco Vela CI, Bosques Padilla FJ. Determination of ammonia concentrations in cirrhosis patients ‐ still confusing after all these years?. Annals of Hepatology 2011;10(Suppl 2):S60‐5. [PUBMED: 22228884] - PubMed
Blanco Vela 2011b
-
- Blanco Vela CI, Poo Ramirez JL. Efficacy of oral L‐ornithine L‐aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Annals of Hepatology 2011;10(Suppl 2):S55‐9. [PUBMED: 22228883] - PubMed
Brown 2006
Brozek 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Hamilton (ON): Grade Working Group, McMaster University, 2008.
Bustamante 1999
-
- Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. Journal of Hepatology 1999;30:890‐5. [PUBMED: 10365817] - PubMed
Butterworth 2014
-
- Butterworth RF. Hepatic encephalopathy in alcoholic cirrhosis. Handbook of Clinical Neurology 2014;125:589‐602. [PUBMED: 25307598] - PubMed
Butterworth 2017
-
- Butterworth RF. L‐ornithine‐L‐aspartate for the treatment of hepatic encephalopathy in cirrhosis: an update on mechanisms of action and therapeutic efficacy. International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) meeting; 2017 Mar 9‐11; New Delhi, India 2017.
Butterworth 2018
Cadranel 2001
-
- Cadranel JF, Lebiez E, Martino V, Bernard B, Koury S, Tourbah A, et al. Focal neurological signs in hepatic encephalopathy in cirrhotic patients: an underestimated entity?. American Journal of Gastroenterology 2001;96:515‐8. [PUBMED: 11232699] - PubMed
Chu 1997
-
- Chu NS, Yang SS, Liaw YF. Evoked potentials in liver diseases. Journal of Gastroenterology and Hepatology 1997;12:S288‐93. [PUBMED: 9407349] - PubMed
Conn 1977
-
- Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, et al. Comparison of lactulose and neomycin in the treatment of chronic portal‐systemic encephalopathy. A double blind controlled trial. Gastroenterology 1977;72:573‐83. [PUBMED: 14049] - PubMed
D'Amico 1986
-
- D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Digestive Diseases and Sciences 1986;31:468‐75. [PUBMED: 3009109] - PubMed
D'Amico 2006
-
- D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44:217‐31. - PubMed
de Jongh 1992
-
- Jongh FE, Janssen HL, Man RA, Hop WC, Schalm SW, Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen‐positive cirrhosis of the liver. Gastroenterology 1992;103:1630‐5. [PUBMED: 1426884] - PubMed
Delcker 2000a
-
- Delcker AM, Jalan R, Schumacher M, Comes G. L‐ornithine‐L‐aspartine vs placebo in the treatment of hepatitis encephalopathy: meta‐analysis of randomised placebo‐controlled trials using individual data. Hepatology (Baltimore, Md.) 2000;32(4 (Part 2)):310A.
Delcker 2000b
-
- Delcker A, Goertelmeyer R, Comes G. Meta‐analysis L‐ornithine L‐aspartate IV. Unpublished Clinical Study Report MRZ 90004‐9902.
Dhiman 1995
-
- Dhiman RK, Saraswat VA, Verma M, Naik SR. Figure connection test: a universal test for assessment of mental state. Journal of Gastroenterology and Hepatology 1995;10:14‐23. [PUBMED: 7620102] - PubMed
Dwan 2008
EASL/AASLD 2014a
-
- American Association for the Study of Liver Diseases, European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. Journal of Hepatology 2014;61:642‐59. [PUBMED: 25015420] - PubMed
EASL/AASLD 2014b
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology (Baltimore, Md.) 2014;60:715‐35. [PUBMED: 25042402] - PubMed
Ferenci 2002
-
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy ‐ definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology (Baltimore, Md.) 2002;35:716‐21. [PUBMED: 11870389] - PubMed
Gebhardt 1997
-
- Gebhardt R, Beckers G, Gaunitz F, Haupt W, Jonitza D, Klein S, et al. Treatment of cirrhotic rats with L‐ornithine‐L‐aspartate enhances urea synthesis and lowers serum ammonia levels. Journal of Pharmacology and Experimental Therapeutics 1997;283:1‐6. [PUBMED: 9336301] - PubMed
Gluud 2017
-
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2017 Issue 6. Art. No.: LIVER.
Groeneweg 1998
-
- Groeneweg M, Quero JC, Bruijn I, Hartmann IJ, Essink‐bot ML, Hop WC, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology (Baltimore, Md.) 1998;28:45‐9. [PUBMED: 9657095] - PubMed
Guérit 2009
-
- Guérit JM, Amantini A, Fischer C, Kaplan PW, Mecarelli O, Schnitzler A, et al. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29:789‐96. [PUBMED: 19638107] - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443‐57. [PUBMED: 16345038] - PubMed
Haussinger 2000
-
- Haussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low‐grade cerebral edema?. Journal of Hepatology 2000;32:1035‐8. [PUBMED: 10898326] - PubMed
Higgins 2008
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997.
Jackson 2016
-
- Jackson CD, Gram M, Halliday E, Olesen SS, Sandberg TH, Drewes AM, et al. New spectral thresholds improve the utility of the electroencephalogram for the diagnosis of hepatic encephalopathy. Clinical Neurophysiology 2016;127:2933‐41. [PUBMED: 27236607] - PubMed
Jepsen 2010
Jiang 2009
-
- Jiang Q, Jiang XH, Zheng MH, Chen YP. L‐ornithine‐L‐aspartate in the management of hepatic encephalopathy: a meta‐analysis. Journal of Gastroenterology and Hepatology 2009;24:9‐14. [PUBMED: 18823442] - PubMed
Kircheis 2002
-
- Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Haussinger D. Critical flicker frequency for quantification of low‐grade hepatic encephalopathy. Hepatology (Baltimore, Md.) 2002;35:357‐66. [PUBMED: 11826409] - PubMed
Kircheis 2007
-
- Kircheis G, Fleig WE, Görtelmeyer R, Grafe S, Häussinger D. Assessment of low‐grade hepatic encephalopathy: a critical appraisal. Journal of Hepatology 2007;47:642‐50. - PubMed
Kircheis 2009
-
- Kircheis G, Knoche A, Hilger N, Manhart F, Schnitzler A, Schulze H, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009;137:1706‐15. [PUBMED: 19686744] - PubMed
Lauridsen 2011
-
- Lauridsen MM, Jepsen P, Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease. Metabolic Brain Disease 2011;26:135‐9. [PUBMED: 21484318] - PubMed
Lockwood 2004
-
- Lockwood AH. Blood ammonia levels and hepatic encephalopathy. Metabolic Brain Disease 2004;19:345‐9. - PubMed
Montagnese 2004
-
- Montagnese S, Amodio P, Morgan MY. Methods for diagnosing hepatic encephalopathy in patients with cirrhosis: a multidimensional approach. Metabolic Brain Disease 2004;19:281‐312. [PUBMED: 15554423] - PubMed
Neff 2010
O'Carroll 1991
-
- O'Carroll RE, Hayes PC, Ebmeier KP, Dougall N, Murray C, Best JJ, et al. Regional cerebral blood flow and cognitive function in patients with chronic liver disease. Lancet 1991;337:1250‐3. [PUBMED: 1674063] - PubMed
Olesen 2016
-
- Olesen SS, Gram M, Jackson CD, Halliday E, Sandberg TH, Drewes AM, et al. Electroencephalogram variability in patients with cirrhosis associates with the presence and severity of hepatic encephalopathy. Journal of Hepatology 2016;65:517‐23. [PUBMED: 27184531] - PubMed
Page 2014
-
- Page MJ, McKenzie JE, Kirkham J, Dwan K, Kramer S, Green S, et al. Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.MR000035.pub2; MR000035] - DOI - PMC - PubMed
Parsons‐Smith 1957
-
- Parsons‐Smith BG, Summerskill WH, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957;273:867‐71. [PUBMED: 13482229] - PubMed
Perez Hernandez 2011
-
- Perez Hernandez JL, Higuera de la Tijera F, Serralde‐Zuniga AE, Abdo Francis JM. Critical analysis of studies evaluating the efficacy of infusion of L‐ornithine L‐aspartate in clinical hepatic encephalopathy in patients with liver failure. Annals of Hepatology 2011;10(Suppl 2):S66‐9. [PUBMED: 22228885] - PubMed
Poodad 2007
-
- Poordad FF. Review article: the burden of hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2007;25(Suppl 1):3‐9. - PubMed
Randolph 2009
-
- Randolph C, Hilsabeck R, Kato A, Kharbanda P, Li YY, Mapelli D, et al. Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29:629‐35. [PUBMED: 19302444] - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roman 2011
-
- Roman E, Córdoba J, Torrens M, Torras X, Villanueva C, Vargas V, et al. Minimal hepatic encephalopathy is associated with falls. American Journal of Gastroenterology 2011;106:476‐82. - PubMed
Rose 1998
-
- Rose C, Michalak A, Pannunzio P, Therrien G, Quack G, Kircheis G, et al. L‐ornithine‐L‐aspartate in experimental portal‐systemic encephalopathy: therapeutic efficacy and mechanism of action. Metabolic Brain Disease 1998;13:147‐57. - PubMed
Rose 1999
-
- Rose C, Michalak A, Rao KV, Quack G, Kircheis G, Butterworth RF. L‐ornithine‐L‐aspartate lowers plasma and cerebrospinal fluid ammonia and prevents brain edema in rats with acute liver failure. Hepatology (Baltimore, Md.) 1999;30:636‐40. [PUBMED: 10462368] - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19:591‐603. - PubMed
Saunders 1981
Savovic 2012
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157:429‐38. [PUBMED: 22945832] - PubMed
Schomerus 1981
-
- Schomerus H, Hamster W, Blunck H, Reinhard U, Mayer K, Dolle W. Latent portasystemic encephalopathy. I. Nature of cerebral functional defects and their effect on fitness to drive. Digestive Diseases and Sciences 1981;26:622‐30. [PUBMED: 7249898] - PubMed
Schomerus 1998
-
- Schomerus H, Hamster W. Neuropsychological aspects of portal‐systemic encephalopathy. Metabolic Brain Disease 1998;13:361‐77. [PUBMED: 10206827] - PubMed
Sharma 2010
-
- Sharma P, Sharma BC, Sarin SK. Predictors of non response to lactulose in patients with cirrhosis and hepatic encephalopathy. European Journal of Gastroenterology & Hepatology 2010;22:526‐31. [PUBMED: 20009938] - PubMed
Soarez 2009
-
- Soarez PC, Oliveira AC, Padovan J, Parise ER, Ferraz MB. A critical analysis of studies assessing L‐ornithine‐L‐aspartate (LOLA) in hepatic encephalopathy treatment. Arquivos de Gastroenterologia 2009;46:241‐7. [PUBMED: 19918694] - PubMed
Sotil 2009
-
- Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transplantation 2009;15:184‐92. - PubMed
Stata 14 [Computer program]
-
- Stata Corp. Stata 14. Texas (USA): Stata Corp, 2007.
Stepanova 2012
-
- Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clinical Gastroenterology and Hepatology 2012;10:1034‐41. [PUBMED: 22642955] - PubMed
Stewart 2007
-
- Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival in patients with end‐stage liver disease. Liver Transplantation 2007;13:1366‐71. - PubMed
Taylor‐Robinson 1994
-
- Taylor‐Robinson SD, Sargentoni J, Marcus CD, Morgan MY, Bryant DJ. Regional variations in cerebral proton spectroscopy in patients with chronic hepatic encephalopathy. Metabolic Brain Disease 1994;9:347‐59. [PUBMED: 7898401] - PubMed
Teasdale 1974
-
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81‐4. [PUBMED: 4136544] - PubMed
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Victor 1965
-
- Victor M, Adams RD, Cole M. The acquired (non‐Wilsonian) type of chronic hepatocerebral degeneration. Medicine 1965;44:345‐96. [PUBMED: 5318075] - PubMed
Weissenborn 1998
-
- Weissenborn K. Diagnosis of encephalopathy. Digestion 1998;59(Suppl 2):22‐4. - PubMed
Weissenborn 2001
-
- Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. Journal of Hepatology 2001;34:768‐73. [PUBMED: 11434627] - PubMed
Wetterslev 2017
Wright 2011
-
- Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver International 2011;31:163‐75. - PubMed
Zacharias 2017
-
- Zacharias HD, Zacharias AP, Ferreira AO, Morgan MY, Gluud LL. Ammonia scavenging agents for people with cirrhosis and hepatic encephalopathy. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012334] - DOI
References to other published versions of this review
Stokes 2016
Yuan 2008
-
- Yuan W, Li J, Xu L, Zhang M, Lu Z, Feng S, et al. L‐ornithine‐L‐aspartate for hepatic encephalopathy. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007344] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

