Discovery of chitin in skeletons of non-verongiid Red Sea demosponges
- PMID: 29763421
- PMCID: PMC5953452
- DOI: 10.1371/journal.pone.0195803
Discovery of chitin in skeletons of non-verongiid Red Sea demosponges
Abstract
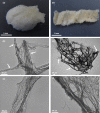
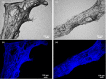
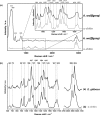
Marine demosponges (Porifera: Demospongiae) are recognized as first metazoans which have developed over millions of years of evolution effective survival strategies based on unique metabolic pathways to produce both biologically active secondary metabolites and biopolymer-based stiff skeletons with 3D architecture. Up to date, among marine demosponges, only representatives of the Verongiida order have been known to synthetize biologically active substances as well as skeletons made of structural polysaccharide chitin. This work, to our knowledge, demonstrates for the first time that chitin is an important structural component within skeletons of non-verongiid demosponges Acarnus wolffgangi and Echinoclathria gibbosa collected in the Red Sea. Calcofluor white staining, FTIR and Raman analysis, ESI-MS, SEM, and fluorescence microscopy as well as a chitinase digestion assay were applied in order to confirm, with strong evidence, the finding of α-chitin in the skeleton of both species. We suggest that, the finding of chitin within these representatives of Poecilosclerida order is a promising step in the evaluation of these sponges as novel renewable sources for both biologically active metabolites and chitin, which are of prospective application for pharmacology and biomedicine.
Conflict of interest statement
Figures






References
-
- Merzendorfer H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur J Cell Biol. 2011;90: 759–769. doi: 10.1016/j.ejcb.2011.04.014 - DOI - PubMed
-
- Deguchi S, Tsujii K, Horikoshi K. In situ microscopic observation of chitin and fungal cells with chitinous cell walls in hydrothermal conditions. Sci Rep. 2015;5: 11907 doi: 10.1038/srep11907 - DOI - PMC - PubMed
-
- Brunner E, Richthammer P, Ehrlich H, Paasch S, Simon P, Ueberlein S, et al. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew Chemie—Int Ed. 2011;48: 9724–9727. - PubMed
-
- Ehrlich H, Krautter M, Hanke T, Simon P, Knieb C, Heinemann S, et al. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). J Exp Zool B Mol Dev Evol. 2007;308: 473–483. doi: 10.1002/jez.b.21174 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

