A Convergence-Based Framework for Cancer Drug Resistance
- PMID: 29763622
- PMCID: PMC5957297
- DOI: 10.1016/j.ccell.2018.03.025
A Convergence-Based Framework for Cancer Drug Resistance
Abstract
Despite advances in cancer biology and therapeutics, drug resistance remains problematic. Resistance is often multifactorial, heterogeneous, and prone to undersampling. Nonetheless, many individual mechanisms of targeted therapy resistance may coalesce into a smaller number of convergences, including pathway reactivation (downstream re-engagement of original effectors), pathway bypass (recruitment of a parallel pathway converging on the same downstream output), and pathway indifference (development of a cellular state independent of the initial therapeutic target). Similar convergences may also underpin immunotherapy resistance. Such parsimonious, convergence-based frameworks may help explain resistance across tumor types and therapeutic categories and may also suggest strategies to overcome it.
Keywords: cancer; drug resistance; heterogeneity; immunotherapy; precision medicine; targeted therapy.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
D.J.K. and C.M.J. declare no competing interests. L.A.G. is a founder of and equity holder in Foundation Medicine and Tango Therapeutics.
Figures
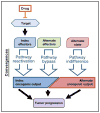

In BRAF-mutant melanoma, MAPK pathway inhibitors target BRAF and its index effectors in the MAPK pathway. Pathway reactivation mechanisms include target alterations (e.g., amplification or alternate splicing), which render BRAF insensitive to drug inhibition, as well as recruitment of upstream, parallel, and downstream effectors, which re-activate index effectors in a BRAF-independent fashion. These mechanisms reactivate the index MAPK pathway effectors downstream of BRAF, conferring resistance to BRAF inhibition.
In prostate cancer, oncogenic signaling through the androgen receptor (AR) is targeted by androgen-deprivation therapy and anti-androgen therapy. Resistance to AR-directed therapy can be mediated by pathway reactivation mechanisms including target alterations, which render AR itself resistant to drug inhibition, and recruitment of upstream effectors (non-gonadal androgens or alternate AR ligands), which reactivate drug-inhibited signaling through AR. In either case, the index oncogenic signaling downstream of AR is re-activated, conferring drug resistance.

In BRAF-mutant melanoma, pathway bypass mechanisms reactivate the core MITF transcriptional output in a manner that is independent of upstream MAPK input. For example, a GPCR-cAMP-CREB signaling axis can substitute for MAPK signaling to sustain an MITF-driven transcriptional program. Likewise, MITF amplification can render MITF itself independent of MAPK signaling.
In prostate cancer, signaling through the glucocorticoid receptor (GR) drives a transcriptional program similar to that of the index AR signaling pathway, rendering cells resistant to inhibition of the AR axis.
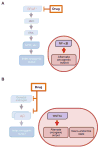
In BRAF-mutant melanoma, the index drug-sensitive transcriptional state is characterized by MAPK-dependent activity of the MITF transcription factor. Resistance to MAPK pathway inhibition can be achieved by transition to an NF-κB-driven, low-MITF transcriptional state that does not require MAPK input for its maintenance.
In prostate cancer, alternative AR-independent cellular states are characterized by Wnt5A-driven and neuroendocrine-like transcriptional programs and are indifferent to inhibition of the AR signaling axis.

Anti-tumor immunity requires a multi-step cycle to achieve immune cell clearance of tumor cells.
Many immunotherapy resistance mechanisms converge on modulation of neoantigen expression, processing, or presentation, thus restoring the index state of escape from anti-tumor immunity.
Alternatively, induction of T cell dysfunction can bypass anti-tumor immunity, either directly (e.g., through alternative checkpoint ligands) or indirectly (e.g., through T regulatory cells or the tumor microenvironment).
Finally, certain oncogenes or malignant cell states may confer an “immune indifferent” resistance state.
References
-
- Amado RG, Wolf M, Peeters M, Cutsem E, Van Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

