Ohio Pediatric Asthma Repository: Opportunities to Revise Care Practices to Decrease Time to Physiologic Readiness for Discharge
- PMID: 29764909
- PMCID: PMC8237236
- DOI: 10.1542/hpeds.2017-0085
Ohio Pediatric Asthma Repository: Opportunities to Revise Care Practices to Decrease Time to Physiologic Readiness for Discharge
Abstract
Background: Large-scale, multisite studies in which researchers evaluate patient- and systems-level factors associated with pediatric asthma exacerbation outcomes are lacking. We sought to investigate patient-level risks and system-level practices related to physiologic readiness for discharge (PRD) in the prospective Ohio Pediatric Asthma Repository.
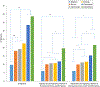
Methods: Participants were children ages 2 to 17 years admitted to an Ohio Pediatric Asthma Repository hospital for asthma exacerbation. Demographics, disease characteristics, and individual hospital practices were collected. The primary outcome was PRD timing (hours from admission or emergency department [ED] presentation until the first 4-hour albuterol spacing).
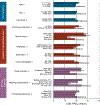
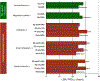
Results: Data for 1005 participants were available (865 ED presentations). Several nonstandard care practices were associated with time to PRD (P < .001). Continuous pulse oximetry was associated with increased time to PRD (P = .004). ED dexamethasone administration was associated with decreased time to PRD (P < .001) and less ICU admittance and intravenous steroid use (P < .0001). Earlier receipt of chest radiograph, antibiotics, and intravenous steroids was associated with shorter time to PRD (P < .05). Care practices associated with shorter time to PRD varied markedly by hospital.
Conclusions: Substantial variation in care practices for inpatient asthma treatment exists among children's hospital systems in Ohio. We found several modifiable, system-level factors and therapies that contribute to PRD that warrant further investigation to identify the best and safest care practices. We also found that there was no standardized measure of exacerbation severity used across the hospitals. The development of such a tool is a critical gap in current practice and is needed to enable definitive comparative effectiveness studies of the management of acute asthma exacerbation.
Copyright © 2018 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Kercsmar reports personal fees from GlaxoSmithKline outside the submitted work; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures

References
-
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Rep. 2011;(32):1–14 - PubMed
-
- Wazeka A, Valacer DJ, Cooper M, Caplan DW, DiMaio M. Impact of a pediatric asthma clinical pathway on hospital cost and length of stay. Pediatr Pulmonol. 2001;32(3):211–216 - PubMed
-
- Shanley LA, Lin H, Flores G. Factors associated with length of stay for pediatric asthma hospitalizations. J Asthma. 2015;52(5):471–477 - PubMed
-
- Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: a systematic review of the literature. Ann Emerg Med. 2000;36(3):181–190 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

