Rapid and Dynamic Alternative Splicing Impacts the Arabidopsis Cold Response Transcriptome
- PMID: 29764987
- PMCID: PMC6096597
- DOI: 10.1105/tpc.18.00177
Rapid and Dynamic Alternative Splicing Impacts the Arabidopsis Cold Response Transcriptome
Abstract
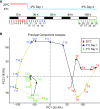
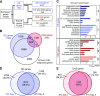
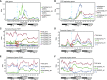
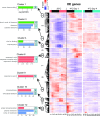
Plants have adapted to tolerate and survive constantly changing environmental conditions by reprogramming gene expression The dynamics of the contribution of alternative splicing (AS) to stress responses are unknown. RNA-sequencing of a time-series of Arabidopsis thaliana plants exposed to cold determines the timing of significant AS changes. This shows a massive and rapid AS response with coincident waves of transcriptional and AS activity occurring in the first few hours of temperature reduction and further AS throughout the cold. In particular, hundreds of genes showed changes in expression due to rapidly occurring AS in response to cold ("early AS" genes); these included numerous novel cold-responsive transcription factors and splicing factors/RNA binding proteins regulated only by AS. The speed and sensitivity to small temperature changes of AS of some of these genes suggest that fine-tuning expression via AS pathways contributes to the thermo-plasticity of expression. Four early AS splicing regulatory genes have been shown previously to be required for freezing tolerance and acclimation; we provide evidence of a fifth gene, U2B"-LIKE Such factors likely drive cascades of AS of downstream genes that, alongside transcription, modulate transcriptome reprogramming that together govern the physiological and survival responses of plants to low temperature.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures
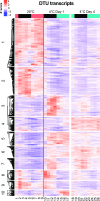
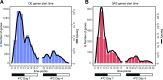
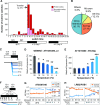
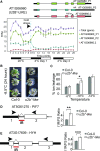

Comment in
-
Alternative Splicing Plays a Major Role in Plant Response to Cold Temperatures.Plant Cell. 2018 Jul;30(7):1378-1379. doi: 10.1105/tpc.18.00430. Epub 2018 Jun 5. Plant Cell. 2018. PMID: 29871987 Free PMC article. No abstract available.
References
-
- Amorim M.F., Willing E.-M., Szabo E.X., Droste-Borel I., Macek B., Schneeberger K., Laubinger S. (2017). Arabidopsis U1 snRNP subunit LUC7 functions in alternative splicing and preferential removal of terminal introns. bioRxiv doi/10.1101/150805.
-
- Barrero-Gil J., Salinas J. (2013). Post-translational regulation of cold acclimation response. Plant Sci. 205-206: 48–54. - PubMed
-
- Barta A., Marquez Y., Brown J.W.S. (2012). Challenges in Plant Alternative Splicing. In Alternative pre-mRNA Splicing: Theory and Protocols, Stamm S., Smith C.W.J., Lührmann R., eds (Weinheim, Germany: Wiley-VCH Verlag; ), pp. 79–91.
Publication types
MeSH terms
Grants and funding
- BB/K006568/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/P009751/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/N022807/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K006835/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M010996/1/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

