Iron-specific Signal Separation from within Heavy Metal Stained Biological Samples Using X-Ray Microtomography with Polychromatic Source and Energy-Integrating Detectors
- PMID: 29765060
- PMCID: PMC5953933
- DOI: 10.1038/s41598-018-25099-z
Iron-specific Signal Separation from within Heavy Metal Stained Biological Samples Using X-Ray Microtomography with Polychromatic Source and Energy-Integrating Detectors
Abstract
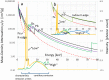
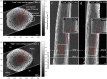
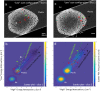
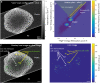
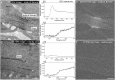
Biological samples are frequently stained with heavy metals in preparation for examining the macro, micro and ultra-structure using X-ray microtomography and electron microscopy. A single X-ray microtomography scan reveals detailed 3D structure based on staining density, yet it lacks both material composition and functional information. Using a commercially available polychromatic X-ray source, energy integrating detectors and a two-scan configuration labelled by their energy- "High" and "Low", we demonstrate how a specific element, here shown with iron, can be detected from a mixture with other heavy metals. With proper selection of scan configuration, achieving strong overlap of source characteristic emission lines and iron K-edge absorption, iron absorption was enhanced enabling K-edge imaging. Specifically, iron images were obtained by scatter plot material analysis, after selecting specific regions within scatter plots generated from the "High" and "Low" scans. Using this method, we identified iron rich regions associated with an iron staining reaction that marks the nodes of Ranvier along nerve axons within mouse spinal roots, also stained with osmium metal commonly used for electron microscopy.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Metscher BD. Biological applications of X-ray microtomography: imaging micro- anatomy, molecular expression and organismal diversity. Microsc. Anal. 2013;27:13–16.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

