Underlying Mechanisms of Cooperativity, Input Specificity, and Associativity of Long-Term Potentiation Through a Positive Feedback of Local Protein Synthesis
- PMID: 29765314
- PMCID: PMC5938377
- DOI: 10.3389/fncom.2018.00025
Underlying Mechanisms of Cooperativity, Input Specificity, and Associativity of Long-Term Potentiation Through a Positive Feedback of Local Protein Synthesis
Abstract
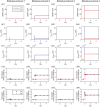
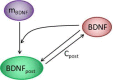
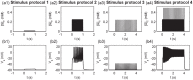
Long-term potentiation (LTP) is a specific form of activity-dependent synaptic plasticity that is a leading mechanism of learning and memory in mammals. The properties of cooperativity, input specificity, and associativity are essential for LTP; however, the underlying mechanisms are unclear. Here, based on experimentally observed phenomena, we introduce a computational model of synaptic plasticity in a pyramidal cell to explore the mechanisms responsible for the cooperativity, input specificity, and associativity of LTP. The model is based on molecular processes involved in synaptic plasticity and integrates gene expression involved in the regulation of neuronal activity. In the model, we introduce a local positive feedback loop of protein synthesis at each synapse, which is essential for bimodal response and synapse specificity. Bifurcation analysis of the local positive feedback loop of brain-derived neurotrophic factor (BDNF) signaling illustrates the existence of bistability, which is the basis of LTP induction. The local bifurcation diagram provides guidance for the realization of LTP, and the projection of whole system trajectories onto the two-parameter bifurcation diagram confirms the predictions obtained from bifurcation analysis. Moreover, model analysis shows that pre- and postsynaptic components are required to achieve the three properties of LTP. This study provides insights into the mechanisms underlying the cooperativity, input specificity, and associativity of LTP, and the further construction of neural networks for learning and memory.
Keywords: associativity; cooperativity; input specificity; local positive feedback; long-term potentiation.
Figures









Similar articles
-
A model of the mechanism of cooperativity and associativity of long-term potentiation in the hippocampus: a fundamental mechanism of associative memory and learning.Biol Cybern. 1991;64(5):365-71. doi: 10.1007/BF00224703. Biol Cybern. 1991. PMID: 2049412
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
-
Cooperativity between hippocampal-prefrontal short-term plasticity through associative long-term potentiation.Brain Res. 2006 Sep 13;1109(1):37-44. doi: 10.1016/j.brainres.2006.06.034. Epub 2006 Jul 21. Brain Res. 2006. PMID: 16859647
-
Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF.Ageing Res Rev. 2004 Nov;3(4):407-30. doi: 10.1016/j.arr.2004.07.002. Ageing Res Rev. 2004. PMID: 15541709 Review.
Cited by
-
Neurobiology of reward-related learning.Neurosci Biobehav Rev. 2021 May;124:224-234. doi: 10.1016/j.neubiorev.2021.02.007. Epub 2021 Feb 10. Neurosci Biobehav Rev. 2021. PMID: 33581225 Free PMC article. Review.
-
Keratan sulfate, an electrosensory neurosentient bioresponsive cell instructive glycosaminoglycan.Glycobiology. 2024 Apr 1;34(3):cwae014. doi: 10.1093/glycob/cwae014. Glycobiology. 2024. PMID: 38376199 Free PMC article. Review.
-
Effects of Exercise on Long-Term Potentiation in Neuropsychiatric Disorders.Adv Exp Med Biol. 2020;1228:439-451. doi: 10.1007/978-981-15-1792-1_30. Adv Exp Med Biol. 2020. PMID: 32342476 Review.
-
Network motifs exhibiting a differential response to spaced and massed inputs.Learn Mem. 2024 Jul 29;31(7):a054012. doi: 10.1101/lm.054012.124. Print 2024 Jul. Learn Mem. 2024. PMID: 39074905 Free PMC article.
-
Mal de Debarquement Syndrome: A Matter of Loops?Front Neurol. 2020 Nov 10;11:576860. doi: 10.3389/fneur.2020.576860. eCollection 2020. Front Neurol. 2020. PMID: 33244308 Free PMC article.
References
-
- Alon U. (2006). An Introduction to System Biology–Design Principles of Biological Circuit. London: Chapman & Hall/CRC.
LinkOut - more resources
Full Text Sources
Other Literature Sources

