Progression and Regression of Hepatic Lesions in a Mouse Model of NASH Induced by Dietary Intervention and Its Implications in Pharmacotherapy
- PMID: 29765319
- PMCID: PMC5938379
- DOI: 10.3389/fphar.2018.00410
Progression and Regression of Hepatic Lesions in a Mouse Model of NASH Induced by Dietary Intervention and Its Implications in Pharmacotherapy
Erratum in
-
Corrigendum: Progression and Regression of Hepatic Lesions in a Mouse Model of NASH Induced by Dietary Intervention and Its Implications in Pharmacotherapy.Front Pharmacol. 2020 Feb 19;11:93. doi: 10.3389/fphar.2020.00093. eCollection 2020. Front Pharmacol. 2020. PMID: 32140108 Free PMC article.
Abstract
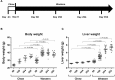
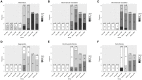
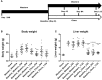
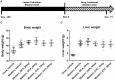
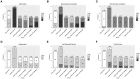
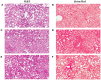
Understanding of the temporal changes of hepatic lesions in the progression and regression of non-alcoholic steatohepatitis (NASH) is vital to elucidation of the pathogenesis of NASH, and critical to the development of a strategy for NASH pharmacotherapy. There are challenges in studying hepatic lesion progression and regression in NASH patients due to the slow development of NASH in humans, one being the requirement for multiple biopsies during the longitudinal follow-up. Here we studied lesion progression and regression in the diet-induced animal model of NASH by application or removal of the pathogenic diet for multiple time periods. Male C57BL/6 mice fed Western diet developed progressive hepatic steatosis/macrovesicular vacuolation, inflammation, and hepatocyte degeneration, as well as perisinusoidal fibrosis and occasionally portal fibrosis as early as 2 months after initiation of the Western diet. In the same period, the mice exhibited elevated ALT (alanine aminotransferase) and AST (aspartate aminotransferase) enzyme activities, CK18 (cytokeratin-18), PIIINP (N-terminal propeptide of type III collagen), and TIMP-1 (tissue inhibitor of metalloproteinase-1). Hepatic steatosis diminished rapidly when the Western diet was replaced by normal rodent chow diet and hepatic inflammation and hepatocyte degeneration were also reduced. Interestingly, perisinusoidal fibrosis and portal fibrosis regressed 8 months after chow diet replacement. To understand pharmacotherapy for NASH, mice with established NASH hepatic lesions were treated with either FXR agonist obeticholic acid (Ocaliva), or CCR2/5 antagonist Cenicriviroc. Similar to the diet replacement, metabolic modulator Ocaliva markedly reduced steatosis/macrovesicular vacuolation, hepatic inflammation, and hepatocyte degeneration effectively, but exhibited no significant effect on liver fibrosis. Anti-inflammation drug Cenicriviroc, on the other hand, markedly decreased inflammation and hepatocyte degeneration, and mildly decreased liver fibrosis, but exhibited no effect on hepatic steatosis/macrovesicular vacuolation. In conclusion, we found the progression of NASH hepatic steatosis/macrovesicular vacuolation, and inflammation eventually lead to hepatocyte death and fibrosis. Life style change and current pharmacotherapies in development may be effective in treating NASH, but their effects on NASH-induced fibrosis may be mild. Since fibrosis is known to be an independent risk for decompensated cirrhosis, cardiovascular events, and mortality, our study suggests that effective anti-fibrosis therapy should be an essential component of the combined pharmacotherapy for advanced NASH.
Keywords: CCR2/5; fibrosis; inflammation; non-alcoholic steatohepatitis; obeticholic acid; pathogenesis; steatosis.
Figures



Similar articles
-
Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice.J Transl Med. 2015 Jun 16;13:193. doi: 10.1186/s12967-015-0552-7. J Transl Med. 2015. PMID: 26077675 Free PMC article.
-
Obeticholic acid protects against hepatocyte death and liver fibrosis in a murine model of nonalcoholic steatohepatitis.Sci Rep. 2018 May 25;8(1):8157. doi: 10.1038/s41598-018-26383-8. Sci Rep. 2018. PMID: 29802399 Free PMC article.
-
A Translational Mouse Model for NASH with Advanced Fibrosis and Atherosclerosis Expressing Key Pathways of Human Pathology.Cells. 2020 Sep 1;9(9):2014. doi: 10.3390/cells9092014. Cells. 2020. PMID: 32883049 Free PMC article.
-
Non-alcoholic steatohepatitis in children.Pediatr Transplant. 2004 Dec;8(6):613-8. doi: 10.1111/j.1399-3046.2004.00241.x. Pediatr Transplant. 2004. PMID: 15598336 Review.
-
Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)-related liver fibrosis: mechanisms, treatment and prevention.Ann Transl Med. 2021 Apr;9(8):729. doi: 10.21037/atm-20-4354. Ann Transl Med. 2021. PMID: 33987427 Free PMC article. Review.
Cited by
-
The traditional Chinese formulae Ling-gui-zhu-gan decoction alleviated non-alcoholic fatty liver disease via inhibiting PPP1R3C mediated molecules.BMC Complement Altern Med. 2019 Jan 7;19(1):8. doi: 10.1186/s12906-018-2424-1. BMC Complement Altern Med. 2019. PMID: 30616587 Free PMC article.
-
Validity of biopsy-based drug effects in a diet-induced obese mouse model of biopsy-confirmed NASH.BMC Gastroenterol. 2019 Dec 28;19(1):228. doi: 10.1186/s12876-019-1149-z. BMC Gastroenterol. 2019. PMID: 31883514 Free PMC article.
-
Collagen co-localized with macrovesicular steatosis better differentiates fibrosis progression in non-alcoholic fatty liver disease mouse models.Front Med (Lausanne). 2023 Jun 2;10:1172058. doi: 10.3389/fmed.2023.1172058. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37332758 Free PMC article.
-
Choline-deficient, high-fat diet-induced MASH in Göttingen Minipigs: characterization and effects of a chow reversal period.Am J Physiol Gastrointest Liver Physiol. 2024 Oct 1;327(4):G571-G585. doi: 10.1152/ajpgi.00120.2024. Epub 2024 Jul 23. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 39041677 Free PMC article.
-
Human translatability of the GAN diet-induced obese mouse model of non-alcoholic steatohepatitis.BMC Gastroenterol. 2020 Jul 6;20(1):210. doi: 10.1186/s12876-020-01356-2. BMC Gastroenterol. 2020. PMID: 32631250 Free PMC article.
References
-
- Angulo P., Kleiner D. E., Dam-Larsen S., Adams L. A., Bjornsson E. S., Charatcharoenwitthaya P., et al. . (2015). Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389.e10–397.e10. 10.1053/j.gastro.2015.04.043 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

