Neutrophils Inhibit Synthesis of Mineralized Extracellular Matrix by Human Bone Marrow-Derived Stromal Cells In Vitro
- PMID: 29765377
- PMCID: PMC5938347
- DOI: 10.3389/fimmu.2018.00945
Neutrophils Inhibit Synthesis of Mineralized Extracellular Matrix by Human Bone Marrow-Derived Stromal Cells In Vitro
Abstract
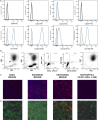
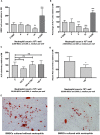
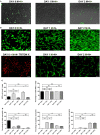
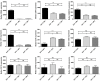
Although controlled local inflammation is essential for adequate bone regeneration, several studies have shown that hyper-inflammatory conditions after major trauma are associated with impaired fracture healing. These hyper-inflammatory conditions include the trauma-induced systemic inflammatory response to major injury, open fractures, and significant injury to the surrounding soft tissues. The current literature suggests that increased or prolonged influx of neutrophils into the fracture hematoma may mediate impairment of bone regeneration after hyper-inflammatory conditions. The underlying mechanism remains unclear. We hypothesize that high neutrophil numbers inhibit synthesis of mineralized extracellular matrix (ECM) by bone marrow stromal cells (BMSCs). We therefore studied the effect of increasing concentrations of neutrophils on ECM synthesis by human BMSCs in vitro. Moreover, we determined how high neutrophil concentrations affect BMSC cell counts, as well as BMSC osteogenic activity determined by alkaline phosphatase (ALP) expression and ALP activity. Co-culture of BMSCs with neutrophils induced a 52% decrease in BMSC cell count (p < 0.01), a 64% decrease in the percentage of ALP+ cells (p < 0.001), a 28% decrease in total ALP activity (p < 0.01), and a significant decrease in the amount of mineralized ECM [38% decrease after 4 weeks (p < 0.05)]. Co-cultures with peripheral blood mononuclear cells and neutrophils within transwells did not induce a significant decrease in ALP activity. In conclusion, our data shows that a decreased amount of mineralized ECM became synthesized by BMSCs, when they were co-cultured with high neutrophil concentrations. Moreover, high neutrophil concentrations induced a decrease in BMSC cell counts and decreased ALP activity. Clarifying the underlying mechanism may contribute to development of therapies that augment bone regeneration or prevent impaired fracture healing after hyper-inflammatory conditions.
Keywords: alkaline phosphatase; bone marrow stromal cell; bone regeneration; fracture healing; multipotent stromal cell; neutrophils; stromal cells.
Figures
Similar articles
-
Endothelial progenitor cells improve the therapeutic effect of mesenchymal stem cell sheets on irradiated bone defect repair in a rat model.J Transl Med. 2018 May 22;16(1):137. doi: 10.1186/s12967-018-1517-4. J Transl Med. 2018. PMID: 29788957 Free PMC article.
-
Bone marrow stromal cells enhance the osteogenic properties of hydroxyapatite scaffolds by modulating the foreign body reaction.J Tissue Eng Regen Med. 2014 Nov;8(11):841-9. doi: 10.1002/term.1574. Epub 2012 Jul 10. J Tissue Eng Regen Med. 2014. PMID: 22782939
-
The influence of proepicardial cells on the osteogenic potential of marrow stromal cells in a three-dimensional tubular scaffold.Biomaterials. 2008 May;29(14):2203-16. doi: 10.1016/j.biomaterials.2008.01.025. Epub 2008 Mar 4. Biomaterials. 2008. PMID: 18289664
-
Extracellular Vesicles Derived from Neutrophils Accelerate Bone Regeneration by Promoting Osteogenic Differentiation of BMSCs.ACS Biomater Sci Eng. 2024 Jun 10;10(6):3868-3882. doi: 10.1021/acsbiomaterials.4c00106. Epub 2024 May 4. ACS Biomater Sci Eng. 2024. PMID: 38703236 Free PMC article.
-
The Neutrophil Life Cycle.Trends Immunol. 2019 Jul;40(7):584-597. doi: 10.1016/j.it.2019.04.013. Epub 2019 May 29. Trends Immunol. 2019. PMID: 31153737 Review.
Cited by
-
Cellular activation status in femoral shaft fracture hematoma following different reaming techniques - A large animal model.J Orthop Res. 2022 Dec;40(12):2822-2830. doi: 10.1002/jor.25309. Epub 2022 Mar 17. J Orthop Res. 2022. PMID: 35301740 Free PMC article.
-
Advances in the application and research of biomaterials in promoting bone repair and regeneration through immune modulation.Mater Today Bio. 2024 Dec 16;30:101410. doi: 10.1016/j.mtbio.2024.101410. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39811613 Free PMC article. Review.
-
Temporal dynamics of immune-stromal cell interactions in fracture healing.Front Immunol. 2024 Feb 22;15:1352819. doi: 10.3389/fimmu.2024.1352819. eCollection 2024. Front Immunol. 2024. PMID: 38455063 Free PMC article. Review.
-
Reliable assessment of bone marrow and bone marrow concentrates using automated hematology analyzer.Regen Med. 2019 Jul;14(7):639-646. doi: 10.2217/rme-2018-0173. Epub 2019 Jul 19. Regen Med. 2019. PMID: 31322050 Free PMC article. Clinical Trial.
-
Recent progress in bone-repair strategies in diabetic conditions.Mater Today Bio. 2023 Oct 20;23:100835. doi: 10.1016/j.mtbio.2023.100835. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 37928253 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

