Suspected unexpected and other adverse reactions to antiretroviral drugs used as post-exposure prophylaxis of HIV infection - five-year experience from clinical practice
- PMID: 29765441
- PMCID: PMC5949906
- DOI: 10.5114/aoms.2016.59701
Suspected unexpected and other adverse reactions to antiretroviral drugs used as post-exposure prophylaxis of HIV infection - five-year experience from clinical practice
Abstract
Introduction: With increased use of antiretroviral drugs (ARVs) in HIV uninfected persons, proper reporting on suspected unexpected serious adverse reactions (SUSARs) and continued insight into adverse drug reactions (ADRs) are needed for adequate information on safety of ARVs in such populations.
Material and methods: Medical documentation of persons receiving ARVs after non-occupational HIV exposure (non-occupational post-exposure prophylaxis - nPEP) during 5 successive years (2009-2013) was evaluated by two HIV physicians. Adverse drug reactions s and SUSARs were defined according to international standards. In statistical analyses Cox proportional hazard models were used to identify independent predictors of developing a first ADR.
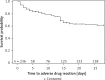
Results: In total 375 persons received nPEP with the following indications: needle stick (43%), unprotected sexual intercourse (17%), rape (10%) and first aid (10%). In 84 (22%) cases the source patient was HIV positive or an active injecting drug user. In total 170 ADRs were reported. One hundred thirty-nine persons had only 1 ADR. The most frequent first ADRs were gastrointestinal disorders (22%), followed by general symptoms (9%), hypersensitivity reactions (1.6%) and CNS symptoms (1.3%). The remaining events represented less than 1% of all patients. Eight (2.1%) patients developed a SUSAR. In multivariate analyses only age at first visit to the clinic was an independent predictor of developing an ADR (HR = 1.17, 95% CI: 1.03-1.34; p = 0.02).
Conclusions: In our observations ADRs in reaction to nPEP were frequent yet usually mild events, mostly occurring in the first 2 weeks and rarely causing discontinuation. The only significant factor increasing the risk of ADR was age. SUSARs were rare, transient and clinically insignificant.
Keywords: HIV prophylaxis; adverse drug reaction; post-exposure prophylaxis; suspected unexpected serious adverse reactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The suspected unexpected and serious adverse events of antiretroviral drugs used as HIV prophylaxis in HIV uninfected persons.J Int AIDS Soc. 2014 Nov 2;17(4 Suppl 3):19733. doi: 10.7448/IAS.17.4.19733. eCollection 2014. J Int AIDS Soc. 2014. PMID: 25397479 Free PMC article.
-
Non-Occupational HIV Post-exposure Prophylaxis: A 10-Year Retrospective Review of Data Following Sexual Exposure From Yaounde Central Hospital, Cameroon.Int J MCH AIDS. 2019;8(2):138-145. doi: 10.21106/ijma.311. Epub 2019 Dec 6. Int J MCH AIDS. 2019. PMID: 31890345 Free PMC article.
-
Monitoring drug safety in Astrakhan, Russia.Int J Risk Saf Med. 2015;27 Suppl 1:S33-4. doi: 10.3233/JRS-150680. Int J Risk Saf Med. 2015. PMID: 26639700
-
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys.Health Technol Assess. 2011 May;15(20):1-234, iii-iv. doi: 10.3310/hta15200. Health Technol Assess. 2011. PMID: 21545758 Review.
-
HIV exposure through contact with body fluids.Prescrire Int. 2012 Apr;21(126):100-1, 103-5. Prescrire Int. 2012. PMID: 22515138 Review.
Cited by
-
Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025.MMWR Recomm Rep. 2025 May 8;74(1):1-56. doi: 10.15585/mmwr.rr7401a1. MMWR Recomm Rep. 2025. PMID: 40331832 Free PMC article.
References
-
- Sonder GJ, van den Hoek A, Regez RM, et al. Trends in HIV postexposure prophylaxis prescription and compliance after sexual exposure in Amsterdam, 2000-2004. Sex Transm Dis. 2007;34:288–93. - PubMed
-
- Lunding S, Katzenstein TL, Kronborg G, et al. The Danish PEP registry: experience with the use of postexposure prophylaxis (PEP) following sexual exposure to HIV from 1998 to 2006. Sex Transm Dis. 2010;37:49–52.. - PubMed
-
- Service USPH Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1–52.. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials