Ringer's lactate solution enhances the inflammatory response during fluid resuscitation of experimentally induced haemorrhagic shock in rats
- PMID: 29765455
- PMCID: PMC5949920
- DOI: 10.5114/aoms.2017.69771
Ringer's lactate solution enhances the inflammatory response during fluid resuscitation of experimentally induced haemorrhagic shock in rats
Abstract
Introduction: Hemorrhagic shock leads to systemic oxygen deficit (hypoxaemia) that results in systemic inflammatory response syndrome (SIRS), a recognised cause of late mortality in this case. The aim of this study was to analyse the impact of fluid resuscitation, using two Ringer solutions, on the microcirculation changes that take place during experimentally induced haemorrhagic shock.
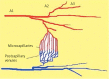
Material and methods: A model of the rat cremaster muscle was used to assess microcirculation in vivo. The experimental groups (n = 10 each) included: control (CTRL); shock (HSG); Ringer's acetate (RAG); and Ringer's lactate (RLG). Microhaemodynamic parameters were measured during the experiment.
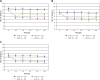
Results: A statistically significantly higher level of leukocytes, both those attached to the endothelium and those located in the extravascular space (p < 0.05), was reported in the lactate Ringer (LR) group compared with the AR group. There were significant differences in the activity of A3 arterioles compared with A1 and A2 arterioles. Ringer's lactate solution seemed to the inflammation response during fluid resuscitation from haemorrhagic shock. A3 arterioles are likely to play a role as a pre-capillary sphincter in the skeletal muscle.
Conclusions: The present study revealed that fluid resuscitation with Ringer's lactate solution exacerbates inflammation in the skeletal muscle. It is worth noting that Ringer's acetate solution reduces local inflammation and could therefore be recommended as the "first line" crystalloid of the fluid resuscitation during haemorrhagic shock.
Keywords: Ringer’s lactate solution; fluid resuscitation; haemorrhagic shock; rat.
Figures












References
-
- Baue AE. MOF, MODS, and SIRS: what is in a name or an acronym? Shock. 2006;26:438–49. - PubMed
-
- Jaeschke H, Bautista AP, Spolarics Z, et al. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–82. - PubMed
-
- Paes-da-Silva F, Gonzalez AP, Tibirica E. Effects of fluid resuscitation on mesenteric microvascular blood flow and lymphatic activity after severe hemorrhagic shock in rats. Shock. 2003;19:55–60. - PubMed
-
- Lee CC, Chang IJ, Yen ZS, et al. Effect of different resuscitation fluids on cytokine response in a rat model of hemorrhagic shock. Shock. 2005;24:177–81. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
