Electrochemically modified Corey-Fuchs reaction for the synthesis of arylalkynes. The case of 2-(2,2-dibromovinyl)naphthalene
- PMID: 29765470
- PMCID: PMC5942384
- DOI: 10.3762/bjoc.14.76
Electrochemically modified Corey-Fuchs reaction for the synthesis of arylalkynes. The case of 2-(2,2-dibromovinyl)naphthalene
Abstract
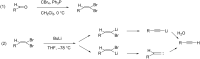
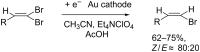
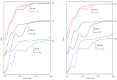
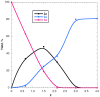
The electrochemical reduction of 2-(2,2-dibromovinyl)naphthalene in a DMF solution (Pt cathode) yields selectively 2-ethynylnaphthalene or 2-(bromoethynyl)naphthalene in high yields, depending on the electrolysis conditions. In particular, by simply changing the working potential and the supporting electrolyte, the reaction can be directed towards the synthesis of the terminal alkyne (Et4NBF4) or the bromoalkyne (NaClO4). This study allowed to establish that 2-(bromoethynyl)naphthalene can be converted into 2-ethynylnaphthalene by cathodic reduction.
Keywords: 2-(2,2-dibromovinyl)naphthalene; Corey–Fuchs reaction; arylalkyne; carbon–bromine bond cleavage; cathodic reduction.
Figures



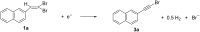


Similar articles
-
Corey-Fuchs reaction enabled synthesis of natural products: a review.RSC Adv. 2025 Mar 17;15(11):8121-8155. doi: 10.1039/d5ra00619h. eCollection 2025 Mar 17. RSC Adv. 2025. PMID: 40103970 Free PMC article. Review.
-
Electrochemical Corey-Winter reaction. Reduction of thiocarbonates in aqueous methanol media and application to the synthesis of a naturally occurring α-pyrone.Beilstein J Org Chem. 2018 Mar 2;14:547-552. doi: 10.3762/bjoc.14.41. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 29623116 Free PMC article.
-
gem-Dibromovinyl boron dipyrrins: synthesis, spectral properties and crystal structures.Dalton Trans. 2019 Aug 14;48(30):11492-11507. doi: 10.1039/c9dt02309g. Epub 2019 Jul 11. Dalton Trans. 2019. PMID: 31292577
-
The competition between cathodic oxygen and ozone reduction and its role in dictating the reaction mechanisms of an electro-peroxone process.Water Res. 2017 Jul 1;118:26-38. doi: 10.1016/j.watres.2017.04.005. Epub 2017 Apr 4. Water Res. 2017. PMID: 28412550
-
[Electrochemically active microorganisms and electrolytically assisted fermentative hydrogen production--a review].Wei Sheng Wu Xue Bao. 2009 Jun;49(6):697-702. Wei Sheng Wu Xue Bao. 2009. PMID: 19673403 Review. Chinese.
Cited by
-
Corey-Fuchs reaction enabled synthesis of natural products: a review.RSC Adv. 2025 Mar 17;15(11):8121-8155. doi: 10.1039/d5ra00619h. eCollection 2025 Mar 17. RSC Adv. 2025. PMID: 40103970 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
