Mechanochemistry of nucleosides, nucleotides and related materials
- PMID: 29765475
- PMCID: PMC5942386
- DOI: 10.3762/bjoc.14.81
Mechanochemistry of nucleosides, nucleotides and related materials
Abstract
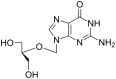
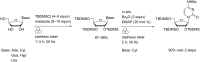
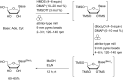
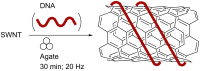
The application of mechanical force to induce the formation and cleavage of covalent bonds is a rapidly developing field within organic chemistry which has particular value in reducing or eliminating solvent usage, enhancing reaction rates and also in enabling the preparation of products which are otherwise inaccessible under solution-phase conditions. Mechanochemistry has also found recent attention in materials chemistry and API formulation during which rearrangement of non-covalent interactions give rise to functional products. However, this has been known to nucleic acids science almost since its inception in the late nineteenth century when Miescher exploited grinding to facilitate disaggregation of DNA from tightly bound proteins through selective denaturation of the latter. Despite the wide application of ball milling to amino acid chemistry, there have been limited reports of mechanochemical transformations involving nucleoside or nucleotide substrates on preparative scales. A survey of these reactions is provided, the majority of which have used a mixer ball mill and display an almost universal requirement for liquid to be present within the grinding vessel. Mechanochemistry of charged nucleotide substrates, in particular, provides considerable benefits both in terms of efficiency (reducing total processing times from weeks to hours) and by minimising exposure to aqueous conditions, access to previously elusive materials. In the absence of large quantities of solvent and heating, side-reactions can be reduced or eliminated. The central contribution of mechanochemistry (and specifically, ball milling) to the isolation of biologically active materials derived from nuclei by grinding will also be outlined. Finally non-covalent associative processes involving nucleic acids and related materials using mechanochemistry will be described: specifically, solid solutions, cocrystals, polymorph transitions, carbon nanotube dissolution and inclusion complex formation.
Keywords: DNA; green chemistry; mechanochemistry; nucleoside; nucleotide.
Figures


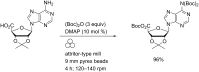

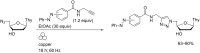






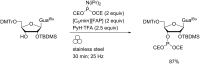



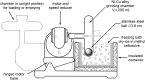

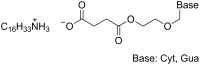
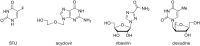
References
-
- McNaught A D, Wilkinson A, editors. IUPAC Compendium of Chemical Technology. 2nd ed. Oxford: Blackwell Scientific Publications; 1997.
-
- Do J-L, Friščić T. Synlett. 2017;28:2066–2092. doi: 10.1055/s-0036-1590854. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
