Ex Vivo Cardiotoxicity of Antineoplastic Casiopeinas Is Mediated through Energetic Dysfunction and Triggered Mitochondrial-Dependent Apoptosis
- PMID: 29765507
- PMCID: PMC5889877
- DOI: 10.1155/2018/8949450
Ex Vivo Cardiotoxicity of Antineoplastic Casiopeinas Is Mediated through Energetic Dysfunction and Triggered Mitochondrial-Dependent Apoptosis
Abstract
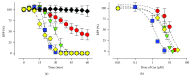
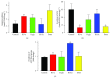
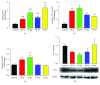
Casiopeinas are a group of copper-based antineoplastic molecules designed as a less toxic and more therapeutic alternative to cisplatin or Doxorubicin; however, there is scarce evidence about their toxic effects on the whole heart and cardiomyocytes. Given this, rat hearts were perfused with Casiopeinas or Doxorubicin and the effects on mechanical performance, energetics, and mitochondrial function were measured. As well, the effects of Casiopeinas-triggered cell death were explored in isolated cardiomyocytes. Casiopeinas III-Ea, II-gly, and III-ia induced a progressive and sustained inhibition of heart contractile function that was dose- and time-dependent with an IC50 of 1.3 ± 0.2, 5.5 ± 0.5, and 10 ± 0.7 μM, correspondingly. Myocardial oxygen consumption was not modified at their respective IC50, although ATP levels were significantly reduced, indicating energy impairment. Isolated mitochondria from Casiopeinas-treated hearts showed a significant loss of membrane potential and reduction of mitochondrial Ca2+ retention capacity. Interestingly, Cyclosporine A inhibited Casiopeinas-induced mitochondrial Ca2+ release, which suggests the involvement of the mitochondrial permeability transition pore opening. In addition, Casiopeinas reduced the viability of cardiomyocytes and stimulated the activation of caspases 3, 7, and 9, demonstrating a cell death mitochondrial-dependent mechanism. Finally, the early perfusion of Cyclosporine A in isolated hearts decreased Casiopeinas-induced dysfunction with reduction of their toxic effect. Our results suggest that heart cardiotoxicity of Casiopeinas is similar to that of Doxorubicin, involving heart mitochondrial dysfunction, loss of membrane potential, changes in energetic metabolites, and apoptosis triggered by mitochondrial permeability.
Figures



Similar articles
-
Antineoplastic copper coordinated complexes (Casiopeinas) uncouple oxidative phosphorylation and induce mitochondrial permeability transition in cardiac mitochondria and cardiomyocytes.J Bioenerg Biomembr. 2016 Feb;48(1):43-54. doi: 10.1007/s10863-015-9640-x. Epub 2016 Jan 7. J Bioenerg Biomembr. 2016. PMID: 26739598
-
Cardiotoxicity of copper-based antineoplastic drugs casiopeinas is related to inhibition of energy metabolism.Toxicol Appl Pharmacol. 2006 Apr 1;212(1):79-88. doi: 10.1016/j.taap.2005.06.023. Epub 2005 Jul 26. Toxicol Appl Pharmacol. 2006. PMID: 16051288
-
The mitochondrial apoptotic pathway is induced by Cu(II) antineoplastic compounds (Casiopeínas®) in SK-N-SH neuroblastoma cells after short exposure times.Biometals. 2017 Feb;30(1):43-58. doi: 10.1007/s10534-016-9983-8. Epub 2016 Dec 17. Biometals. 2017. PMID: 27988860
-
Drug-induced mitochondrial dysfunction and cardiotoxicity.Am J Physiol Heart Circ Physiol. 2015 Nov;309(9):H1453-67. doi: 10.1152/ajpheart.00554.2015. Epub 2015 Sep 18. Am J Physiol Heart Circ Physiol. 2015. PMID: 26386112 Free PMC article. Review.
-
[Cardiotoxicity risk assessment of anticancer drugs by focusing on mitochondrial quality of human iPS cell-derived cardiomyocytes].Nihon Yakurigaku Zasshi. 2025;160(1):9-12. doi: 10.1254/fpj.24056. Nihon Yakurigaku Zasshi. 2025. PMID: 39756913 Review. Japanese.
Cited by
-
Metallodrugs in the battle against non-small cell lung cancer: unlocking the potential for improved therapeutic outcomes.Front Pharmacol. 2023 Aug 31;14:1242488. doi: 10.3389/fphar.2023.1242488. eCollection 2023. Front Pharmacol. 2023. PMID: 37727388 Free PMC article.
-
Amorphous SiO2 nanoparticles promote cardiac dysfunction via the opening of the mitochondrial permeability transition pore in rat heart and human cardiomyocytes.Part Fibre Toxicol. 2020 May 7;17(1):15. doi: 10.1186/s12989-020-00346-2. Part Fibre Toxicol. 2020. PMID: 32381100 Free PMC article.
-
The Bulk Breast Cancer Cell and Breast Cancer Stem Cell Activity of Binuclear Copper(II)-Phenanthroline Complexes.Chemistry. 2023 Aug 10;29(45):e202301188. doi: 10.1002/chem.202301188. Epub 2023 Jul 13. Chemistry. 2023. PMID: 37249243 Free PMC article.
-
Intracellular ATP Concentration and Implication for Cellular Evolution.Biology (Basel). 2021 Nov 12;10(11):1166. doi: 10.3390/biology10111166. Biology (Basel). 2021. PMID: 34827159 Free PMC article.
-
Anticancer activity, DNA binding and cell mechanistic studies of estrogen-functionalised Cu(II) complexes.J Biol Inorg Chem. 2020 Feb;25(1):49-60. doi: 10.1007/s00775-019-01732-8. Epub 2019 Oct 26. J Biol Inorg Chem. 2020. PMID: 31655896
References
-
- Schwartz R. G., McKenzie W. B., Alexander J., et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. The American Journal of Medicine. 1987;82(6):1109–1118. doi: 10.1016/0002-9343(87)90212-9. - DOI - PubMed
-
- Doroshow J. H., Davies K. J. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. The Journal of Biological Chemistry. 1986;261(7):3068–3074. - PubMed
-
- Bravo-Gomez M. E., Garcia-Ramos J. C., Gracia-Mora I., Ruiz-Azuara L. Antiproliferative activity and QSAR study of copper(II) mixed chelate [Cu(N–N)(acetylacetonato)]NO3 and [Cu(N–N)(glycinato)]NO3 complexes, (Casiopeínas®) Journal of Inorganic Biochemistry. 2009;103(2):299–309. doi: 10.1016/j.jinorgbio.2008.10.006. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

