Crystal structure of De-hydro-dieugenol B methyl ether, a neolignan from Nectandra leucantha Nees and Mart (Lauraceae)
- PMID: 29765758
- PMCID: PMC5946980
- DOI: 10.1107/S2056989018003717
Crystal structure of De-hydro-dieugenol B methyl ether, a neolignan from Nectandra leucantha Nees and Mart (Lauraceae)
Abstract
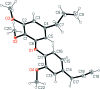
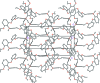
In the title compound, C21H24O4 (systematic name: 4,5'-diallyl-2,2',3'-tri-meth-oxy-diphenyl ether), the aromatic rings lie almost perpendicular to each other [dihedral angle = 85.96 (2)°]. The allyl side chains show similar configurations, with Car-C-C=C (ar = aromatic) torsion angles of -123.62 (12) and -115.54 (12)°. A possible weak intra-molecular C-H⋯O inter-action is observed. In the crystal, mol-ecules are connected by two C-H⋯O hydrogen bonds, forming undulating layers lying parallel to the bc plane. Weak C-H⋯π and π-π stacking inter-actions also occur.
Keywords: crystal structure; eugenol; neolignan.
Figures
References
-
- Agilent (2014). CrysAlis PRO. Agilent Technologies, Yarnton, England.
-
- Fernandes, T. S., Copetti, D., do Carmo, G., Neto, A. T., Pedroso, M., Silva, U. F., Mostardeiro, M. A., Burrow, R., Dalcol, I. I. & Morel, A. F. (2017). Phytochemistry, 141, 131–139. - PubMed
-
- Gottlieb, O. R. (1972). Phytochemistry, 11, 1537–1570.
-
- Grecco, S. S., Costa-Silva, T. A., Jerz, G., de Sousa, F. S., Alves Conserva, G. A., Mesquita, J. T., Galuppo, M. K., Tempone, A. G., Neves, B. J., Andrade, C. H., Cunha, R. O. L., Uemi, M., Sartorelli, P. & Lago, J. H. G. (2017). Phytomedicine, 24, 62–67. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
