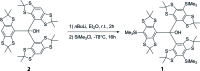Tris[2,2,6,6-tetra-methyl-8-(tri-methyl-sil-yl)benzo[1,2- d;4,5- d']bis-(1,3-di-thiol)-4-yl]methanol diethyl ether monosolvate
- PMID: 29765762
- PMCID: PMC5946984
- DOI: 10.1107/S2056989018004516
Tris[2,2,6,6-tetra-methyl-8-(tri-methyl-sil-yl)benzo[1,2- d;4,5- d']bis-(1,3-di-thiol)-4-yl]methanol diethyl ether monosolvate
Abstract
The title compound, a tri-aryl-methanol, C46H64OS12Si31, was synthesized via li-thia-tion of tris-2,2,6,6-tetra-methyl-benzo[1,2-d;4,5-d']bis-[1,3]di-thiol-4-yl-methanol, 2, and electrophilic quenching with tri-methyl-silyl chloride. The current crystal structure reveals information about the reactivity of this compound and compares well with the structure reported for the unsubstituted parent compound 2 [Driesschaert et al. (2012 ▸). Eur. J. Org. Chem.33, 6517-6525]. The title compound 1 forms mol-ecular propellers and crystallizes in P [Formula: see text], featuring an unusually long Si-Car bond of 1.910 (3) Å. Moreover, the geometry at the central quaternary carbon is rather trigonal-pyramidal than tetra-hedral due to vast intra-molecular stress. One tri-methyl-silyl group is disordered over two positions in a 0.504 (4):0.496 (4) ratio and one S atom is disordered over two positions in a 0.509 (7):0.491 (7) ratio. The contribution of disordered diethyl ether solvent mol-ecule(s) was removed using the PLATON SQUEEZE (Spek, 2015 ▸) solvent masking procedure. These solvent mol-ecules are not considered in the given chemical formula and other crystal data.
Keywords: EPR; crystal structure; spin label; trityl radical; triarylmethanol.
Figures
References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1.
-
- Bondi, A. (1964). J. Phys. Chem. 68, 441–451.
-
- Bruker (2015). SADABS, SMART and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Decroos, C., Prangé, T., Mansuy, D., Boucher, J. & Li, Y. (2011). Chem. Commun. 47, 4805–4807. - PubMed
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous