Select nutrients and their effects on conceptus development in mammals
- PMID: 29767122
- PMCID: PMC5945975
- DOI: 10.1016/j.aninu.2015.07.005
Select nutrients and their effects on conceptus development in mammals
Abstract
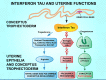
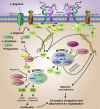
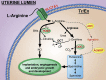
The dialogue between the mammalian conceptus (embryo/fetus and associated membranes) involves signaling for pregnancy recognition and maintenance of pregnancy during the critical peri-implantation period of pregnancy when the stage is set for implantation and placentation that precedes fetal development. Uterine epithelial cells secrete and/or transport a wide range of molecules, including nutrients, collectively referred to as histotroph that are transported into the fetal-placental vascular system to support growth and development of the conceptus. The availability of uterine-derived histotroph has long-term consequences for the health and well-being of the fetus and the prevention of adult onset of metabolic diseases. Histotroph includes numerous amino acids, but arginine plays a particularly important role as a source of nitric oxide and polyamines required for fetal-placental development in rodents, swine and humans through mechanisms that remain to be fully elucidated. Mechanisms whereby arginine regulates expression of genes via the mechanistic target of rapamycin cell signaling pathways critical to conceptus development, implantation and placentation are discussed in detail in this review.
Keywords: Amino acids; Conceptus development; Interferon tau; Pregnancy; Secreted phosphoprotein 1; Trophectoderm.
Figures


Similar articles
-
Amino acids and conceptus development during the peri-implantation period of pregnancy.Adv Exp Med Biol. 2015;843:23-52. doi: 10.1007/978-1-4939-2480-6_2. Adv Exp Med Biol. 2015. PMID: 25956294 Review.
-
Interferon tau: Influences on growth and development of the conceptus.Theriogenology. 2020 Jul 1;150:75-83. doi: 10.1016/j.theriogenology.2020.01.069. Epub 2020 Feb 19. Theriogenology. 2020. PMID: 32088030
-
Uterine histotroph and conceptus development: select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewes.Biol Reprod. 2011 Dec;85(6):1094-107. doi: 10.1095/biolreprod.111.094722. Epub 2011 Aug 24. Biol Reprod. 2011. PMID: 21865556 Review.
-
The many faces of interferon tau.Amino Acids. 2015 Mar;47(3):449-60. doi: 10.1007/s00726-014-1905-x. Epub 2015 Jan 4. Amino Acids. 2015. PMID: 25557050 Review.
-
Functional role of arginine during the peri-implantation period of pregnancy. II. Consequences of loss of function of nitric oxide synthase NOS3 mRNA in ovine conceptus trophectoderm.Biol Reprod. 2014 Sep;91(3):59. doi: 10.1095/biolreprod.114.121202. Epub 2014 Jul 24. Biol Reprod. 2014. PMID: 25061098
Cited by
-
Cerebrum, liver, and muscle regulatory networks uncover maternal nutrition effects in developmental programming of beef cattle during early pregnancy.Sci Rep. 2021 Feb 2;11(1):2771. doi: 10.1038/s41598-021-82156-w. Sci Rep. 2021. PMID: 33531552 Free PMC article.
-
Maternal periconceptual nutrition, early pregnancy, and developmental outcomes in beef cattle.J Anim Sci. 2020 Dec 1;98(12):skaa358. doi: 10.1093/jas/skaa358. J Anim Sci. 2020. PMID: 33165531 Free PMC article. Review.
-
Early steps of embryo implantation are regulated by exchange of extracellular vesicles between the embryo and the endometrium.FASEB J. 2022 Aug;36(8):e22450. doi: 10.1096/fj.202200677R. FASEB J. 2022. PMID: 35848638 Free PMC article.
-
Conceptus metabolomic profiling reveals stage-specific phenotypes leading up to pregnancy recognition in cattle†.Biol Reprod. 2021 May 7;104(5):1022-1033. doi: 10.1093/biolre/ioab021. Biol Reprod. 2021. PMID: 33590828 Free PMC article.
-
Temporal and spatial expression of adrenomedullin and its receptors in the porcine uterus and peri-implantation conceptuses.Biol Reprod. 2021 Oct 11;105(4):876-891. doi: 10.1093/biolre/ioab110. Biol Reprod. 2021. PMID: 34104954 Free PMC article.
References
-
- Albertini D.F., Overstrom E.W., Ebert K.M. Changes in the organization of the actin cytoskeleton during preimplantation development of the pig embryo. Biol Reprod. 1987;37:441–451. - PubMed
-
- Amoroso E.C. Placentation. In: Parkes A.S., editor. 3rd ed. vol. 2. Longmans Green; London: 1952. pp. 127–311. (Marshall׳s physiology of reproduction).
-
- Bazer F.W., Burghardt R.C., Johnson G.A., Spencer T.E., Wu G. Interferons and progesterone for establishment and maintenance of pregnancy: interactions among novel cell signaling pathways. Reprod Biol. 2008;8:179–211. - PubMed
-
- Bazer F.W., Robison O.W., Clawson A.J., Ulberg L.C. Uterine capacity at two stages of gestation in gilts following embryo superinduction. J Anim Sci. 1969;29:30–34. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

