Comparison of the synergistic anticancer activity of AlPcS4 photodynamic therapy in combination with different low‑dose chemotherapeutic agents on gastric cancer cells
- PMID: 29767247
- PMCID: PMC6059740
- DOI: 10.3892/or.2018.6438
Comparison of the synergistic anticancer activity of AlPcS4 photodynamic therapy in combination with different low‑dose chemotherapeutic agents on gastric cancer cells
Abstract
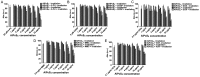
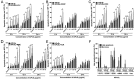
Limited cellular delivery and internalization efficiency of Al(III) phthalocyanine chloride tetrasulfonic acid (AlPcS4) induce poor penetration ability in cells and a slight photodynamic therapy (PDT) effect on gastric cancer. The combination treatment of AlPcS4/PDT with low‑dose chemotherapeutic agents may provide a promising treatment strategy to increase the weak delivery efficiency of AlPcS4, reducing the dose of chemical agents without reducing efficacy, and improving apoptosis‑inducing abilities, thereby increasing the antitumor effects and decreasing the noxious side effects on gastric cancer. We investigated and compared the synergistic antitumor growth effect on gastric cancer cells by combining AlPcS4/PDT treatment with different low‑dose chemotherapeutic agents, namely, 5‑fluorouracil (5‑FU), doxorubicin (DOX), cisplatin (CDDP), mitomycin C (MMC), and vincristine (VCR). The inhibitory effect was increased in treatments that combined AlPcS4/PDT with all the aforementioned low‑dose chemotherapeutic agents, to a different extent. An evident synergistic effect was obtained in the combination treatment of AlPcS4/PDT with low‑dose 5‑FU, DOX, and MMC by increasing AlPcS4 intracellular uptake ability, improving apoptosis‑inducing abilities, and prolonging apoptosis‑inducing time. The low‑dose chemotherapeutic agents prolonged the apoptosis‑inducing period of AlPcS4/PDT, and AlPcS4/PDT quickly improved apoptosis‑inducing abilities of chemotherapy even at low doses. Generally, the combination treatment of AlPcS4/PDT with low‑dose chemotherapeutic agents had significant antitumor growth effects in addition to a low dark‑cytotoxicity effect on gastric cancer, thereby representing an effective and feasible therapy method for gastric cancer.
Figures








Similar articles
-
AlPcS4-PDT for gastric cancer therapy using gold nanorod, cationic liposome, and Pluronic® F127 nanomicellar drug carriers.Int J Nanomedicine. 2018 Apr 4;13:2017-2036. doi: 10.2147/IJN.S154054. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29670347 Free PMC article.
-
Nanosystem Integrated with Photosensitizer and Novel Targeting Chemotherapy Agent for Gastric Cancer Chemo-Photodynamic Combined Therapy.J Biomed Nanotechnol. 2018 Aug 1;14(8):1430-1447. doi: 10.1166/jbn.2018.2598. J Biomed Nanotechnol. 2018. PMID: 29903058
-
Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells.Photodiagnosis Photodyn Ther. 2019 Dec;28:88-97. doi: 10.1016/j.pdpdt.2019.08.027. Epub 2019 Aug 24. Photodiagnosis Photodyn Ther. 2019. PMID: 31454716
-
Phthalocyanine induced phototherapy coupled with Doxorubicin; a promising novel treatment for breast cancer.Expert Rev Anticancer Ther. 2017 Aug;17(8):693-702. doi: 10.1080/14737140.2017.1347505. Epub 2017 Jul 3. Expert Rev Anticancer Ther. 2017. PMID: 28657372 Review.
-
PDT for Gastric Cancer - the view from China.Photodiagnosis Photodyn Ther. 2023 Jun;42:103366. doi: 10.1016/j.pdpdt.2023.103366. Epub 2023 Feb 24. Photodiagnosis Photodyn Ther. 2023. PMID: 36841280 Review.
Cited by
-
The "Light Knife" for Gastric Cancer: Photodynamic Therapy.Pharmaceutics. 2022 Dec 28;15(1):101. doi: 10.3390/pharmaceutics15010101. Pharmaceutics. 2022. PMID: 36678730 Free PMC article. Review.
-
Photoactive metabolite mediated photodynamic therapy of Rhabdomyosarcoma cell lines using medicinal plants and Doxorubicin co-treatments.BMC Complement Med Ther. 2024 Jul 15;24(1):270. doi: 10.1186/s12906-024-04575-2. BMC Complement Med Ther. 2024. PMID: 39010043 Free PMC article.
-
CD44 Targeting Mediated by Polymeric Nanoparticles and Combination of Chlorine TPCS2a-PDT and Docetaxel-Chemotherapy for Efficient Killing of Breast Differentiated and Stem Cancer Cells In Vitro.Cancers (Basel). 2020 Jan 23;12(2):278. doi: 10.3390/cancers12020278. Cancers (Basel). 2020. PMID: 31979218 Free PMC article.
-
Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives.Cancers (Basel). 2020 Sep 3;12(9):2491. doi: 10.3390/cancers12092491. Cancers (Basel). 2020. PMID: 32899137 Free PMC article. Review.
-
Potential of Cyanine Derived Dyes in Photodynamic Therapy.Pharmaceutics. 2021 May 31;13(6):818. doi: 10.3390/pharmaceutics13060818. Pharmaceutics. 2021. PMID: 34072719 Free PMC article. Review.
References
-
- Vrouenraets MB, Visser GW, Snow GB, van Dongen GA. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003;23:505–522. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

