Reliability of In Vitro Methods Used to Measure Intrinsic Clearance of Hydrophobic Organic Chemicals by Rainbow Trout: Results of an International Ring Trial
- PMID: 29767801
- PMCID: PMC6061802
- DOI: 10.1093/toxsci/kfy113
Reliability of In Vitro Methods Used to Measure Intrinsic Clearance of Hydrophobic Organic Chemicals by Rainbow Trout: Results of an International Ring Trial
Abstract
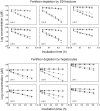
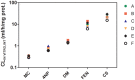
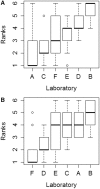
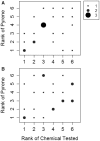
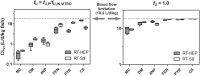
In vitro assays are widely employed to obtain intrinsic clearance estimates used in toxicokinetic modeling efforts. However, the reliability of these methods is seldom reported. Here we describe the results of an international ring trial designed to evaluate two in vitro assays used to measure intrinsic clearance in rainbow trout. An important application of these assays is to predict the effect of biotransformation on chemical bioaccumulation. Six laboratories performed substrate depletion experiments with cyclohexyl salicylate, fenthion, 4-n-nonylphenol, deltamethrin, methoxychlor, and pyrene using cryopreserved hepatocytes and liver S9 fractions from trout. Variability within and among laboratories was characterized as the percent coefficient of variation (CV) in measured in vitro intrinsic clearance rates (CLIN VITRO, INT; ml/h/mg protein or 106 cells) for each chemical and test system. Mean intralaboratory CVs for each test chemical averaged 18.9% for hepatocytes and 14.1% for S9 fractions, whereas interlaboratory CVs (all chemicals and all tests) averaged 30.1% for hepatocytes and 22.4% for S9 fractions. When CLIN VITRO, INT values were extrapolated to in vivo intrinsic clearance estimates (CLIN VIVO, INT; l/d/kg fish), both assays yielded similar levels of activity (<4-fold difference for all chemicals). Hepatic clearance rates (CLH; l/d/kg fish) calculated using data from both assays exhibited even better agreement. These findings show that both assays are highly reliable and suggest that either may be used to inform chemical bioaccumulation assessments for fish. This study highlights several issues related to the demonstration of assay reliability and may provide a template for evaluating other in vitro biotransformation assays.
Figures


References
-
- Arnot J. A., Gobas F. A. P. C. (2003). A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb. Sci. 22, 337–345.
-
- Arnot J. A., Gobas F. A. P. C. (2004). A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environ. Toxicol. Chem. 23, 2343–2355. - PubMed
-
- Bischof I., Koster J., Segner H., Schlechtriem C. (2016). Hepatocytes as in vitro test system to investigate metabolite patterns of pesticides in farmed rainbow trout and common carp: Comparison between in vivo and in vitro and across species. Comp. Biochem. Physiol. 187, 62–73. - PubMed
-
- Brinkman M., Schlechtriem C., Reininghaus M., Eichbaum K., Buchinger S., Reifferscheid G., Hollert H., Preuss T. G. (2016). Cross-species extrapolation of uptake and disposition of neutral organic chemicals in fish using a multispecies physiologically-based toxicokinetic model framework. Environ. Sci. Technol. 50, 1914–1923. - PubMed
-
- Chen Y., Hermens J. L. M., Jonker M. T. O., Arnot J. A., Armitage J. M., Brown T., Nichols J. W., Fay K. A., Droge S. T. J. (2016). Which molecular features affect the intrinsic hepatic clearance rate of ionizable chemicals in fish? Environ. Sci. Technol. 50, 12722–12731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

