Targeting the Latent Reservoir for HIV-1
- PMID: 29768175
- PMCID: PMC6196732
- DOI: 10.1016/j.immuni.2018.04.030
Targeting the Latent Reservoir for HIV-1
Abstract
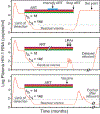
Antiretroviral therapy can effectively block HIV-1 replication and prevent or reverse immunodeficiency in HIV-1-infected individuals. However, viral replication resumes within weeks of treatment interruption. The major barrier to a cure is a small pool of resting memory CD4+ T cells that harbor latent HIV-1 proviruses. This latent reservoir is now the focus of an intense international research effort. We describe how the reservoir is established, challenges involved in eliminating it, and pharmacologic and immunologic strategies for targeting this reservoir. The development of a successful cure strategy will most likely require understanding the mechanisms that maintain HIV-1 proviruses in a latent state and pathways that drive the proliferation of infected cells, which slows reservoir decay. In addition, a cure will require the development of effective immunologic approaches to eliminating infected cells. There is renewed optimism about the prospect of a cure, and the interventions discussed here could pave the way.
Copyright © 2018. Published by Elsevier Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

