GSK3β Regulates Brain Energy Metabolism
- PMID: 29768193
- PMCID: PMC6082412
- DOI: 10.1016/j.celrep.2018.04.045
GSK3β Regulates Brain Energy Metabolism
Abstract
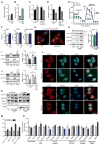
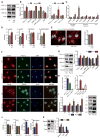
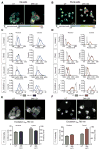
GSK3β is a serine threonine kinase implicated in the progression of Alzheimer's disease. Although the role of GSK3β in growth and pathology has been extensively studied, little is known about the metabolic consequences of GSK3β manipulation, particularly in the brain. Here, we show that GSK3β regulates mitochondrial energy metabolism in human H4 neuroglioma cells and rat PC12-derived neuronal cells and that inhibition of GSK3β in mice in vivo alters metabolism in the hippocampus in a region-specific manner. We demonstrate that GSK3β inhibition increases mitochondrial respiration and membrane potential and alters NAD(P)H metabolism. These metabolic effects are associated with increased PGC-1α protein stabilization, enhanced nuclear localization, and increased transcriptional co-activation. In mice treated with the GSK3β inhibitor lithium carbonate, changes in hippocampal energy metabolism are linked to increased PGC-1α. These data highlight a metabolic role for brain GSK3β and suggest that the GSK3β/PGC-1α axis may be important in neuronal metabolic integrity.
Keywords: GSK3β; PGC-1α; brain; energy metabolism; glia; hippocampus; lithium; neuron.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. - PubMed
-
- Bird DK, Yan L, Vrotsos KM, Eliceiri KW, Vaughan EM, Keely PJ, White JG, Ramanujam N. Metabolic mapping of MCF10A human breast cells via multiphoton fluorescence lifetime imaging of the coenzyme NADH. Cancer Res. 2005;65:8766–8773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

