Pembrolizumab-induced acute thrombosis: A case report
- PMID: 29768369
- PMCID: PMC5976311
- DOI: 10.1097/MD.0000000000010772
Pembrolizumab-induced acute thrombosis: A case report
Abstract
Rationale: Acute thrombosis has not been reported in the literature so far in lung cancer patients as an immune-related adverse event (irAE) associated with PD-1 pathway inhibitors.
Patients concerns: Here, we for the first time present two NSCLC (non-small cell lung cancer) patients suffering from acute thrombosis as a pembrolizumab-induced irAE. Immediate treatment with continuous heparin infusion improved their symptoms and enabled them to continue pembrolizumab administration.
Methods: Ethical approval was given by the ethics committee of Osaka International Cancer Institute and the informed consents were given by the patients.
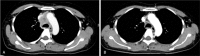
Diagnosis: Serum D-dimer level testing, venous ultrasonography, enhanced computed tomography (CT).
Interventions: Continuous heparin infusion, direct oral anticoagulants (DOAC).
Outcomes: Immediate continuous heparin infusion improved their symptoms and continuing pembrolizumab with direct oral anticoagulant successfully induced tumor shrinkage.
Lessons: Reinvigoration of exhausted T cells by pembrolizumab induced systemic inflammation possibly resulting in development of thrombosis. Although acute thrombosis is a rare irAE, it may lead to cessation of treatment and can be lethal.
Conflict of interest statement
Conflicts of Interest and Source of Funding: Dr Imamura reports personal fees from Ono pharmaceutical co., Ltd, personal fees from MSD, personal fees from Bristol-Myers Squibb, outside the submitted work. Other authors have no COI.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

