Tissue-specific activities of the Fat1 cadherin cooperate to control neuromuscular morphogenesis
- PMID: 29768404
- PMCID: PMC5973635
- DOI: 10.1371/journal.pbio.2004734
Tissue-specific activities of the Fat1 cadherin cooperate to control neuromuscular morphogenesis
Abstract
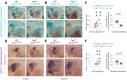
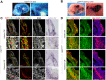
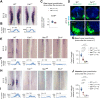
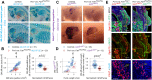
Muscle morphogenesis is tightly coupled with that of motor neurons (MNs). Both MNs and muscle progenitors simultaneously explore the surrounding tissues while exchanging reciprocal signals to tune their behaviors. We previously identified the Fat1 cadherin as a regulator of muscle morphogenesis and showed that it is required in the myogenic lineage to control the polarity of progenitor migration. To expand our knowledge on how Fat1 exerts its tissue-morphogenesis regulator activity, we dissected its functions by tissue-specific genetic ablation. An emblematic example of muscle under such morphogenetic control is the cutaneous maximus (CM) muscle, a flat subcutaneous muscle in which progenitor migration is physically separated from the process of myogenic differentiation but tightly associated with elongating axons of its partner MNs. Here, we show that constitutive Fat1 disruption interferes with expansion and differentiation of the CM muscle, with its motor innervation and with specification of its associated MN pool. Fat1 is expressed in muscle progenitors, in associated mesenchymal cells, and in MN subsets, including the CM-innervating pool. We identify mesenchyme-derived connective tissue (CT) as a cell type in which Fat1 activity is required for the non-cell-autonomous control of CM muscle progenitor spreading, myogenic differentiation, motor innervation, and for motor pool specification. In parallel, Fat1 is required in MNs to promote their axonal growth and specification, indirectly influencing muscle progenitor progression. These results illustrate how Fat1 coordinates the coupling of muscular and neuronal morphogenesis by playing distinct but complementary actions in several cell types.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








References
-
- Francius C, Clotman F. Generating spinal motor neuron diversity: a long quest for neuronal identity. Cell Mol Life Sci. 2014;71(5):813–29. Epub 2013/06/15. doi: 10.1007/s00018-013-1398-x . - DOI - PMC - PubMed
-
- Comai G, Tajbakhsh S. Molecular and cellular regulation of skeletal myogenesis. Curr Top Dev Biol. 2014;110:1–73. Epub 2014/09/25. doi: 10.1016/B978-0-12-405943-6.00001-4 . - DOI - PubMed
-
- Buckingham M, Rigby PW. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell. 2014;28(3):225–38. Epub 2014/02/15. doi: 10.1016/j.devcel.2013.12.020 . - DOI - PubMed
-
- Vasyutina E, Birchmeier C. The development of migrating muscle precursor cells. Anat Embryol (Berl). 2006;211 Suppl 1:37–41. Epub 2006/09/16. doi: 10.1007/s00429-006-0118-9 . - DOI - PubMed
-
- Chevallier A, Kieny M. On the role of the connective tissue in the patterning of the chick limb musculature. Wilehm Roux Arch Dev Biol. 1982;191(4):277–80. Epub 1982/07/01. doi: 10.1007/BF00848416 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

