Secretion of autoimmune antibodies in the human subcutaneous adipose tissue
- PMID: 29768501
- PMCID: PMC5955545
- DOI: 10.1371/journal.pone.0197472
Secretion of autoimmune antibodies in the human subcutaneous adipose tissue
Abstract
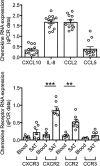
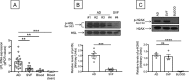
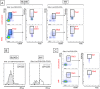
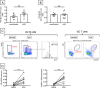
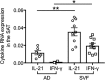
The adipose tissue (AT) contributes to systemic and B cell intrinsic inflammation, reduced B cell responses and secretion of autoimmune antibodies. In this study we show that adipocytes in the human obese subcutaneous AT (SAT) secrete several pro-inflammatory cytokines and chemokines, which contribute to the establishment and maintenance of local and systemic inflammation, and consequent suboptimal immune responses in obese individuals, as we have previously shown. We also show that pro-inflammatory chemokines recruit immune cells expressing the corresponding receptors to the SAT, where they also contribute to local and systemic inflammation, secreting additional pro-inflammatory mediators. Moreover, we show that the SAT generates autoimmune antibodies. During the development of obesity, reduced oxygen and consequent hypoxia and cell death lead to further release of pro-inflammatory cytokines, "self" protein antigens, cell-free DNA and lipids. All these stimulate class switch and the production of autoimmune IgG antibodies which have been described to be pathogenic. In addition to hypoxia, we have measured cell cytotoxicity and DNA damage mechanisms, which may also contribute to the release of "self" antigens in the SAT. All these processes are significantly elevated in the SAT as compared to the blood. We definitively found that fat-specific IgG antibodies are secreted by B cells in the SAT and that B cells express mRNA for the transcription factor T-bet and the membrane marker CD11c, both involved in the production of autoimmune IgG antibodies. Finally, the SAT also expresses RNA for cytokines known to promote Germinal Center formation, isotype class switch, and plasma cell differentiation. Our results show novel mechanisms for the generation of autoimmune antibody responses in the human SAT and allow the identification of new pathways to possibly manipulate in order to reduce systemic inflammation and autoantibody production in obese individuals.
Conflict of interest statement
Figures





References
-
- Apovian CM, Gokce N. Obesity and cardiovascular disease. Circulation. 2012;125(9):1178–82. doi: 10.1161/CIRCULATIONAHA.111.022541 ; PubMed Central PMCID: PMCPMC3693443. - DOI - PMC - PubMed
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485 . - DOI - PubMed
-
- Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152(4):673–84. doi: 10.1016/j.cell.2013.01.041 . - DOI - PubMed
-
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069 ; PubMed Central PMCID: PMCPMC1483173. - DOI - PMC - PubMed
-
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

