Adenosine: Synthetic Methods of Its Derivatives and Antitumor Activity
- PMID: 29769005
- PMCID: PMC6327119
- DOI: 10.2174/1389557518666180516163539
Adenosine: Synthetic Methods of Its Derivatives and Antitumor Activity
Abstract
Since 1929, several researchers have conducted studies in relation to the nucleoside of adenosine (1) mainly distribution identifying, characterizing their biological importance and synthetic chemistry to which this type of molecule has been subjected to obtain multiple of its derivatives. The receptors that interact with adenosine and its derivatives, called purinergic receptors, are classified as A1, A2A, A2B and A3. In the presence of agonists and antagonists, these receptors are involved in various physiological processes and diseases. This review describes and compares some of the synthetic methods that have been developed over the last 30 years for obtaining some adenosine derivatives, classified according to substitution processes, complexation, mating and conjugation. Finally, we mention that although the concentrations of these nucleosides are low in normal tissues, they can increase rapidly in pathophysiological conditions such as hypoxia, ischemia, inflammation, trauma and cancer. In particular, the evaluation of adenosine derivatives as adjunctive therapy promises to have a significant impact on the treatment of certain cancers, although the transfer of these results to clinical practice requires a deeper understanding of how adenosine regulates the process of tumorigenesis.
Keywords: Adenosine; adenosine derivatives; adenosine receptors; complexing; conjugation; glioblastoma; substitution..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures




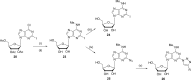



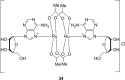

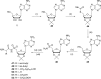
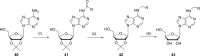



References
-
- Gessi S., Merighi S., Sacchetto V., Simioni C., Borea P.A. Adenosine receptors and cancer. Biochim. Biophys. Acta. 2011;1808(5):1400–1412. - PubMed
-
- Cosyn L. Thesis submitted to the faculty of pharmaceutical sciences to obtain the degree of doctor in pharmaceutical sciences. Ghent University, 2008. Synthesis and biological evaluation of purine and pyrimidine ligands for the a3 andp2y2 purinergic receptors. .
-
- Volpini R., Costanzi S., Lambertucci C., Taffi S., Vittori S., Klotz K-N.N. 6-alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A3 receptor and a starting point for searching A2B ligands. J. Med. Chem. 2002;45(15):3271–3279. - PubMed
-
- Antonioli L., Blandizzi C., Pacher P., Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer. 2013;13(12):842–857. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

