A complex culturally targeted intervention to reduce Hispanic disparities in living kidney donor transplantation: an effectiveness-implementation hybrid study protocol
- PMID: 29769080
- PMCID: PMC5956564
- DOI: 10.1186/s12913-018-3151-5
A complex culturally targeted intervention to reduce Hispanic disparities in living kidney donor transplantation: an effectiveness-implementation hybrid study protocol
Abstract
Background: The shortage of organs for kidney transplantation for patients with end-stage renal disease (ESRD) is magnified in Hispanics/Latin Americans in the United States. Living donor kidney transplantation (LDKT) is the treatment of choice for ESRD. However, compared to their representation on the transplant waitlist, fewer Hispanics receive a LDKT than non-Hispanic whites. Barriers to LDKT for Hispanics include: lack of knowledge, cultural concerns, and language barriers. Few interventions have been designed to reduce LDKT disparities. This study aims to reduce Hispanic disparities in LDKT through a culturally targeted intervention.
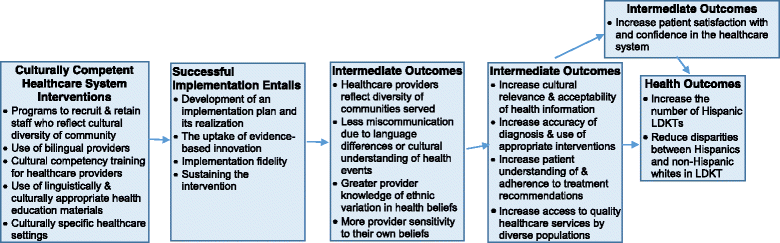
Methods/design: Using a prospective effectiveness-implementation hybrid design involving pre-post intervention evaluation with matched controls, we will implement a complex culturally targeted intervention at two transplant centers in Dallas, TX and Phoenix, AZ. The goal of the study is to evaluate the effect of Northwestern Medicine's® Hispanic Kidney Transplant Program's (HKTP) key culturally targeted components (outreach, communication, education) on Hispanic LDKT rates over five years. The main hypothesis is that exposure to the HKTP will reduce disparities by increasing the ratio of Hispanic to non-Hispanic white LDKTs and the number of Hispanic LDKTs. We will also examine other process and outcome measures including: dialysis patient outreach, education session attendance, marketing efforts, Hispanic patients added to the waitlist, Hispanic potential donors per potential recipient, and satisfaction with culturally competent care. We will use mixed methods based on the Promoting Action on Research Implementation in Health Services (revised PARIHS) and the Consolidated Framework for Implementation Research (CFIR) frameworks to formatively evaluate the fidelity and innovative adaptations to HKTP's components at both study sites, to identify moderating factors that most affect implementation fidelity, and to identify adaptations that positively and negatively affect outcomes for patients.
Discussion: Our study will provide new knowledge about implementing culturally targeted interventions and their impact on reducing health disparities. Moreover, the study of a complex organizational-level intervention's implementation over five years is rare in implementation science; as such, this study is poised to contribute new knowledge to the factors influencing how organizational-level interventions are sustained over time.
Trial registration: (ClinicalTrials.gov registration # NCT03276390 , date of registration: 9-7-17, retrospectively registered).
Keywords: Complex interventions; End-stage kidney disease; Equity; Health disparities; Hybrid study; Implementation science; Organizational-level intervention.
Conflict of interest statement
Ethics approval and consent to participate
This manuscript does not report on the use of any human or animal data or tissue. However, the study has received Institutional Review Board approval from Northwestern University (#STU00201331) on 10–29-15, and subsequently from BUMC, MC, UC, and HM. Written informed consent will be obtained from all faculty, staff, and administrators participating in site visit one-on-one interviews, and verbal consent will be obtained for group discussions and surveys. Written informed consent will be obtained from all potential transplant recipients and when applicable, their accompanying family members, to review their medical records, to be audio-recorded during transplant education sessions, and to complete CAHPS surveys. Verbal consent will be obtained from patients to audio record their telephone conversations with scheduler staff. A waiver of consent will be obtained from BUMC and MC to retrospectively review medical records of potential recipients and potential living donors, and to prospectively review potential living donors’ medical records. No identifying information of study participants will be shared with the transplant teams. All identifying information will be kept separate from the datasets in digital and paper forms to ensure confidentiality. Data collected from all study sites will be de-identified for analysis purposes, then submitted online to a confidential computer database stored on a server, REDCap, that is kept and maintained at Northwestern University, to which only Dr. Gordon and her Research Coordinators will have access. REDCap is a secure, web-based application for building and managing online data capture for research studies. Digital audio recordings will be stored in secure servers at Northwestern. Electronic documents will be compressed into a .zip file using basic encryption and a password known only to Dr. Gordon and research staff. Study participants completing CAHPS surveys will be compensated $20 for their time.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- U.S. Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016.
-
- OPTN/UNOS. U.S. transplants performed : January 1, 1988 - August 31, 2017. Based on OPTN data as of September 21, 2017. Website accessed 24 Sept 2017. URL (https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/%23).
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical