Inflammatory pattern of the infrapatellar fat pad in dogs with canine cruciate ligament disease
- PMID: 29769086
- PMCID: PMC5956839
- DOI: 10.1186/s12917-018-1488-y
Inflammatory pattern of the infrapatellar fat pad in dogs with canine cruciate ligament disease
Abstract
Background: Despite the importance of inflammation during the pathogenesis of cranial cruciate ligament disease (CCLD) in dogs and despite the latest knowledge suggesting a significant role of adipose tissue in osteoarthritis, the infrapatellar fat pad (IFP) was up to now mostly disregarded in veterinary investigations. In the present study, the inflammatory activity of the IFP, the main adipose structure within the stifle joint, was thoroughly investigated to evaluate its potential impact in the pathogenesis of this common disease of our canine companions. Samples of IFP, subcutaneous adipose tissue (ScAT) of the thigh and synovial fluid in both diseased (n = 36) and healthy control (n = 23) dogs were tested for their immune cell composition but also for interleukins (IL-1β, IL-6, IL-8, IL-10), degradative enzymes (MMP-1, MMP-3, MMP-13, TIMP-2, iNOS) and adipokines (leptin and adiponectin). Characterization of the immune cell composition was ascertained by fluorescence activated cell sorting. Gene expression and protein release of the inflammatory markers was determined by real RT-qPCR and ELISA.
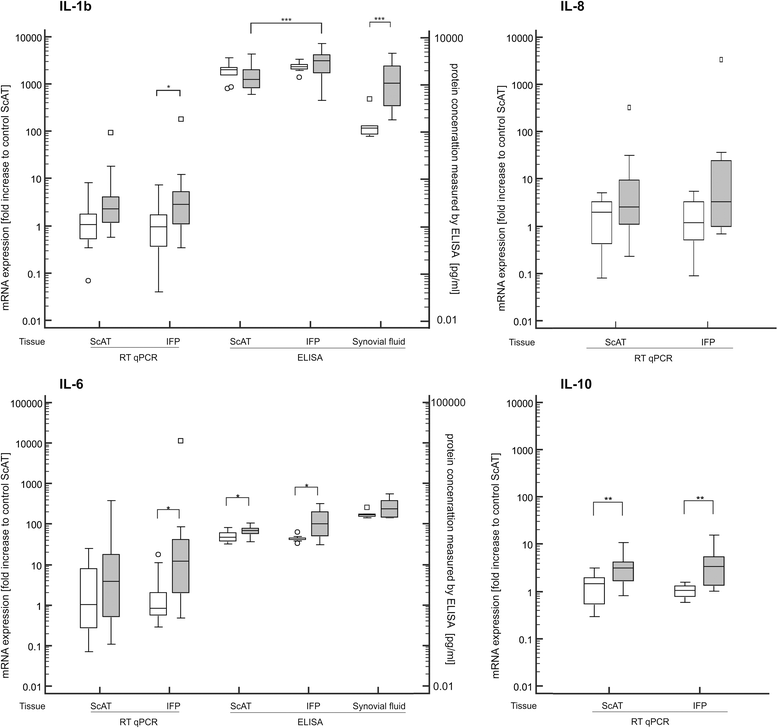
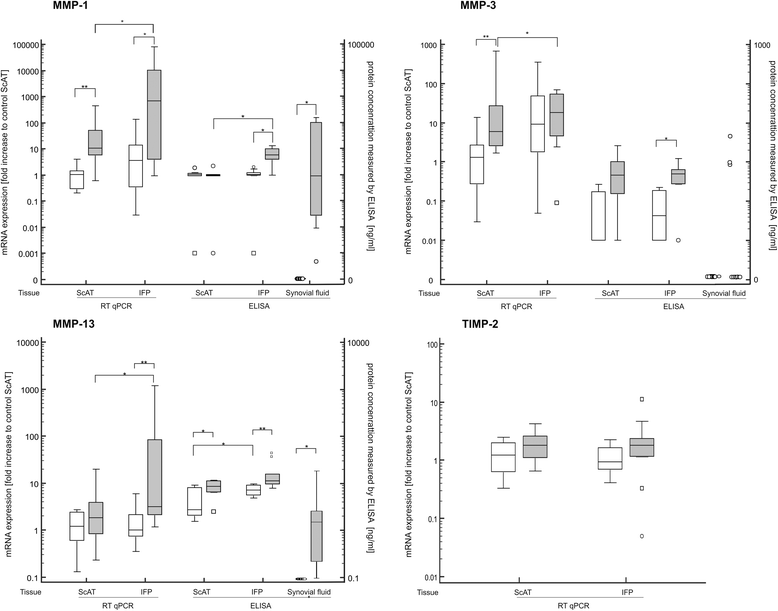
Results: IFPs of dogs with CCLD had a significantly increased immune cell count with T cells (CD3) as the most abundant immune cells. T cells and macrophages (CD14) were significantly increased compared to healthy controls or corresponding ScAT. In addition, IFPs of dogs with CCLD demonstrated a significant increase on gene as well as protein level of multiple inflammatory indicators (IL-1β, IL-6, MMP-1, MMP-13) compared to the other tissues. TNFα was only increased on gene expression. Adipokine analysis showed higher secretion of adiponectin and lower leptin secretion in IFP from dogs with CCLD than from controls. In the synovial fluid from dogs with CCLD concentrations of IL-1β, MMP-1, MMP-13 as well as leptin were significantly increased compared to the synovial fluid from healthy control dogs.
Conclusions: The present study indicates that the IFP is a potential contributory factor in the pathogenesis of CCLD, due to its inflammatory phenotype and the proximity within the stifle joint. To determine the extent of this possible inter-relationship, further studies need to be undertaken.
Keywords: CCLD; Cranial cruciate ligament disease; Dogs; Inflammatory pattern; Infrapatellar fat pad; Osteoarthritis.
Conflict of interest statement
Ethics approval and consent to participate
All procedures were approved by the commission of animal experimentation of the Canton of Berne and Basel, Switzerland (BE116/13; BS2197). Prior to study enrolment, written owner consent was obtained for each dog.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Bennett D, Tennant B, Lewis DG, Baughan J, May C, Carter SA. Reappraisal of anterior cruciate ligament disease in the dog. J Small Anim Pract. 1988;295:275–297. doi: 10.1111/j.1748-5827.1988.tb02286.x. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

