Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC)
- PMID: 29769148
- PMCID: PMC5956851
- DOI: 10.1186/s40425-018-0349-3
Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC)
Abstract
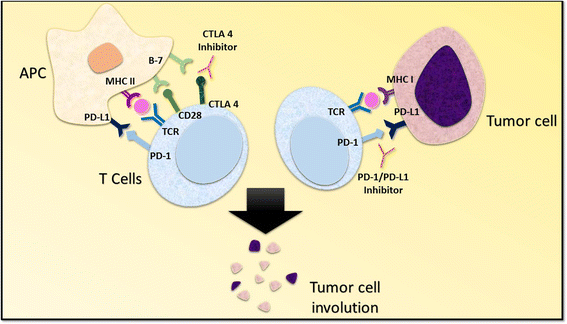
Immunotherapy is among the most rapidly evolving treatment strategies in oncology. The therapeutic potential of immune-checkpoint inhibitors is exemplified by the recent hail of Food and Drug Administration (FDA) approvals for their use in various malignancies. Continued efforts to enhance outcomes with immunotherapy agents have led to the formulation of advanced treatment strategies. Recent evidence from pre-clinical studies evaluating immune-checkpoint inhibitors in various cancer cell-lines has suggested that combinatorial approaches may have superior survival outcomes compared to single-agent immunotherapy regimens. Preliminary trials assessing combination therapy with anti-PD-1/PD-L1 plus anti-CTLA-4 immune-checkpoint inhibitors have documented considerable advantages in survival indices over single-agent immunotherapy. The therapeutic potential of combinatorial approaches is highlighted by the recent FDA approval of nivolumab plus ipilimumab for patients with advanced melanoma. Presently, dual-immune checkpoint inhibition with anti-programmed death receptor-1/programmed cell death receptor- ligand-1 (anti-PD-1/PD-L1) plus anti-cytotoxic T lymphocyte associated antigen-4 (anti-CTLA-4) monoclonal antibodies (MoAbs) is being evaluated for a wide range of tumor histologies. Furthermore, several ongoing clinical trials are investigating combination checkpoint inhibition in association with traditional treatment modalities such as chemotherapy, surgery, and radiation. In this review, we summarize the current landscape of combination therapy with anti-PD-1/PD-L1 plus anti-CTLA-4 MoAbs for patients with melanoma and non-small cell lung cancer (NSCLC). We present a synopsis of the prospects for expanding the indications of dual immune-checkpoint inhibition therapy to a more diverse set of tumor histologies.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
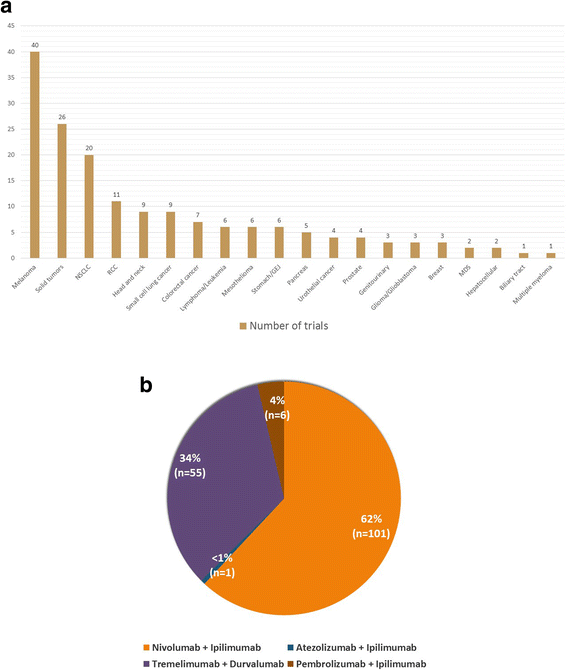
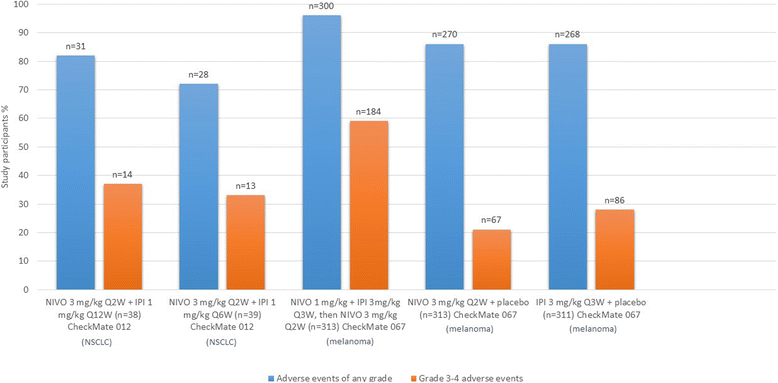
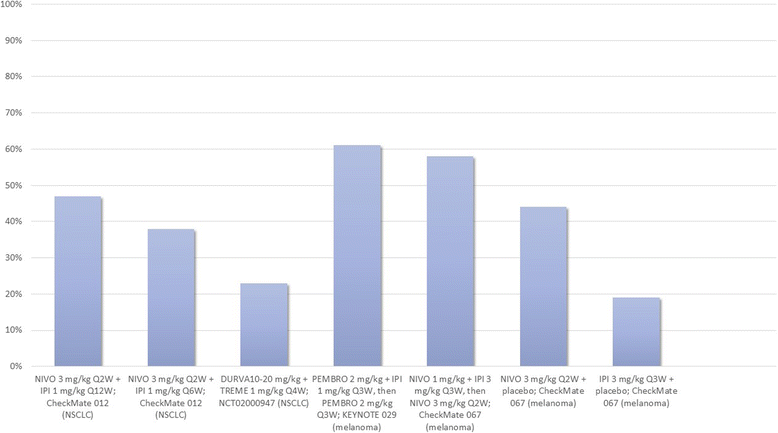
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
