Cancer-Associated Fibroblasts Drive Glycolysis in a Targetable Signaling Loop Implicated in Head and Neck Squamous Cell Carcinoma Progression
- PMID: 29769197
- PMCID: PMC6050074
- DOI: 10.1158/0008-5472.CAN-17-1076
Cancer-Associated Fibroblasts Drive Glycolysis in a Targetable Signaling Loop Implicated in Head and Neck Squamous Cell Carcinoma Progression
Abstract
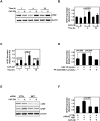
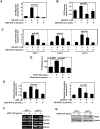
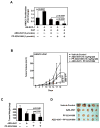
Despite aggressive therapies, head and neck squamous cell carcinoma (HNSCC) is associated with a less than 50% 5-year survival rate. Late-stage HNSCC frequently consists of up to 80% cancer-associated fibroblasts (CAF). We previously reported that CAF-secreted HGF facilitates HNSCC progression; however, very little is known about the role of CAFs in HNSCC metabolism. Here, we demonstrate that CAF-secreted HGF increases extracellular lactate levels in HNSCC via upregulation of glycolysis. CAF-secreted HGF induced basic FGF (bFGF) secretion from HNSCC. CAFs were more efficient than HNSCC in using lactate as a carbon source. HNSCC-secreted bFGF increased mitochondrial oxidative phosphorylation and HGF secretion from CAFs. Combined inhibition of c-Met and FGFR significantly inhibited CAF-induced HNSCC growth in vitro and in vivo (P < 0.001). Our cumulative findings underscore reciprocal signaling between CAF and HNSCC involving bFGF and HGF. This contributes to metabolic symbiosis and a targetable therapeutic axis involving c-Met and FGFR.Significance: HNSCC cancer cells and CAFs have a metabolic relationship where CAFs secrete HGF to induce a glycolytic switch in HNSCC cells and HNSCC cells secrete bFGF to promote lactate consumption by CAFs. Cancer Res; 78(14); 3769-82. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures




References
-
- Kim L, King T, Agulnik M. Head and neck cancer: changing epidemiology and public health implications. Oncology. 2010;24(10):915–9. 24. - PubMed
-
- Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Seminars in oncology. 2014;41(2):217–34. - PubMed
-
- Knowles LM, Stabile LP, Egloff AM, Rothstein ME, Thomas SM, Gubish CT, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(11):3740–50. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

