Microbial Interactions in the Cystic Fibrosis Airway
- PMID: 29769279
- PMCID: PMC6062800
- DOI: 10.1128/JCM.00354-18
Microbial Interactions in the Cystic Fibrosis Airway
Abstract
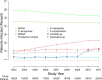
Interactions in the airway ecology of cystic fibrosis may alter organism persistence and clinical outcomes. Better understanding of such interactions could guide clinical decisions. We used generalized estimating equations to fit logistic regression models to longitudinal 2-year patient cohorts in the Cystic Fibrosis Foundation Patient Registry, 2003 to 2011, in order to study associations between the airway organisms present in each calendar year and their presence in the subsequent year. Models were adjusted for clinical characteristics and multiple observations per patient. Adjusted models were tested for sensitivity to cystic fibrosis-specific treatments. The study included 28,042 patients aged 6 years and older from 257 accredited U.S. care centers and affiliates. These patients had produced sputum specimens for at least two consecutive years that were cultured for methicillin-sensitive Staphylococcus aureus, methicillin-resistant S. aureus, Pseudomonas aeruginosa, Burkholderia cepacia complex, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, and Candida and Aspergillus species. We analyzed 99.8% of 538,458 sputum cultures from the patients during the study period. Methicillin-sensitive S. aureus was negatively associated with subsequent Paeruginosa. Paeruginosa was negatively associated with subsequent B. cepacia complex, Axylosoxidans, and Smaltophilia. Bcepacia complex was negatively associated with the future presence of all bacteria studied, as well as with that of Aspergillus species. Paeruginosa, B. cepacia complex, and S. maltophilia were each reciprocally and positively associated with Aspergillus species. Independently of patient characteristics, the organisms studied interact and alter the outcomes of treatment decisions, sometimes in unexpected ways. By inhibiting P. aeruginosa, methicillin-sensitive S. aureus may delay lung disease progression. Paeruginosa and B. cepacia complex may inhibit other organisms by decreasing airway biodiversity, potentially worsening lung disease.
Keywords: Burkholderia; Pseudomonas aeruginosa; Staphylococcus aureus; airway infections; cystic fibrosis; logistic regression; microbial ecology.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Cystic Fibrosis Foundation. 2016. Cystic Fibrosis Foundation Patient Registry 2015. Annual data report Cystic Fibrosis Foundation, Bethesda, MD.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

