Repeated exposure of epithelial cells to apoptotic cells induces the specific selection of an adaptive phenotype: Implications for tumorigenesis
- PMID: 29769319
- PMCID: PMC6028949
- DOI: 10.1074/jbc.RA117.001290
Repeated exposure of epithelial cells to apoptotic cells induces the specific selection of an adaptive phenotype: Implications for tumorigenesis
Abstract
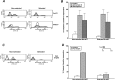
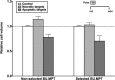
The consequences of apoptosis extend beyond the mere death of the cell. We have shown that receptor-mediated recognition of apoptotic target cells by viable kidney proximal tubular epithelial cells (PTECs) inhibits PTEC proliferation, growth, and survival. Here, we tested the hypothesis that continual exposure to apoptotic targets can induce a phenotypic change in responding PTECs, as in other instances of natural selection. In particular, we demonstrate that repeated exposure to apoptotic targets leads to emergence of a PTEC line (denoted BU.MPTSEL) resistant to apoptotic target-induced death. Resistance is exquisitely specific. Not only are BU.MPTSEL responders fully resistant to apoptotic target-induced death (∼85% survival versus <10% survival of nonselected cells) but do so while retaining sensitivity to all other target-induced responses, including inhibition of proliferation and growth. Moreover, the resistance of BU.MPTSEL responders is specific to target-induced apoptosis, as apoptosis in response to other suicidal stimuli occurs normally. Comparison of the signaling events induced by apoptotic target exposure in selected versus nonselected responders indicated that the acquired resistance of BU.MPTSEL cells lies in a regulatory step affecting the generation of the pro-apoptotic protein, truncated BH3 interacting-domain death agonist (tBID), most likely at the level of BID cleavage by caspase-8. This specific adaptation has especial relevance for cancer, in which the prominence and persistence of cell death entail magnification of the post-mortem effects of apoptotic cells. Just as cancer cells acquire specific resistance to chemotherapeutic agents, we propose that cancer cells may also adapt to their ongoing exposure to apoptotic targets.
Keywords: B-cell lymphoma 2 (Bcl-2) family; apoptosis; cancer biology; cell death; cell proliferation; cell signaling; directed evolution; epithelial cell; innate immunity; tumor microenvironment.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures










Similar articles
-
Apoptotic cells activate AMP-activated protein kinase (AMPK) and inhibit epithelial cell growth without change in intracellular energy stores.J Biol Chem. 2015 Sep 11;290(37):22352-69. doi: 10.1074/jbc.M115.667345. Epub 2015 Jul 16. J Biol Chem. 2015. PMID: 26183782 Free PMC article.
-
Recognition-dependent signaling events in response to apoptotic targets inhibit epithelial cell viability by multiple mechanisms: implications for non-immune tissue homeostasis.J Biol Chem. 2012 Apr 20;287(17):13761-77. doi: 10.1074/jbc.M112.350843. Epub 2012 Mar 6. J Biol Chem. 2012. PMID: 22396534 Free PMC article.
-
Recognition of apoptotic cells by epithelial cells: conserved versus tissue-specific signaling responses.J Biol Chem. 2010 Jan 15;285(3):1829-40. doi: 10.1074/jbc.M109.018440. Epub 2009 Nov 12. J Biol Chem. 2010. PMID: 19910463 Free PMC article.
-
Present and potential future adjuvant issues in high-grade astrocytic glioma treatment.Adv Tech Stand Neurosurg. 2009;34:3-35. doi: 10.1007/978-3-211-78741-0_1. Adv Tech Stand Neurosurg. 2009. PMID: 19368079 Review.
-
Exploitation of Apoptotic Regulation in Cancer.Front Immunol. 2018 Feb 27;9:241. doi: 10.3389/fimmu.2018.00241. eCollection 2018. Front Immunol. 2018. PMID: 29535707 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

