O-GlcNAc transferase missense mutations linked to X-linked intellectual disability deregulate genes involved in cell fate determination and signaling
- PMID: 29769320
- PMCID: PMC6036218
- DOI: 10.1074/jbc.RA118.002583
O-GlcNAc transferase missense mutations linked to X-linked intellectual disability deregulate genes involved in cell fate determination and signaling
Abstract
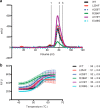
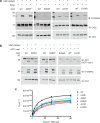
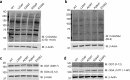
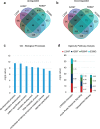
It is estimated that ∼1% of the world's population has intellectual disability, with males affected more often than females. OGT is an X-linked gene encoding for the enzyme O-GlcNAc transferase (OGT), which carries out the reversible addition of N-acetylglucosamine (GlcNAc) to Ser/Thr residues of its intracellular substrates. Three missense mutations in the tetratricopeptide (TPR) repeats of OGT have recently been reported to cause X-linked intellectual disability (XLID). Here, we report the discovery of two additional novel missense mutations (c.775 G>A, p.A259T, and c.1016 A>G, p.E339G) in the TPR domain of OGT that segregate with XLID in affected families. Characterization of all five of these XLID missense variants of OGT demonstrates modest declines in thermodynamic stability and/or activities of the variants. We engineered each of the mutations into a male human embryonic stem cell line using CRISPR/Cas9. Investigation of the global O-GlcNAc profile as well as OGT and O-GlcNAc hydrolase levels by Western blotting showed no gross changes in steady-state levels in the engineered lines. However, analyses of the differential transcriptomes of the OGT variant-expressing stem cells revealed shared deregulation of genes involved in cell fate determination and liver X receptor/retinoid X receptor signaling, which has been implicated in neuronal development. Thus, here we reveal two additional mutations encoding residues in the TPR regions of OGT that appear causal for XLID and provide evidence that the relatively stable and active TPR variants may share a common, unelucidated mechanism of altering gene expression profiles in human embryonic stem cells.
Keywords: RNA-seq; XLID; enzyme kinetics; genetic disease; glycosyltransferase; O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT); O-linked N-acetylglucosamine (O-GlcNAc); intellectual disability; post-translational modification; transcriptomics.
© 2018 Selvan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Shafi R., Iyer S. P., Ellies L. G., O'Donnell N., Marek K. W., Chui D., Hart G. W., and Marth J. D. (2000) The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc. Natl. Acad. Sci. U.S.A. 97, 5735–5739 10.1073/pnas.100471497 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

