Targeting BCL-2 regulated apoptosis in cancer
- PMID: 29769323
- PMCID: PMC5990650
- DOI: 10.1098/rsob.180002
Targeting BCL-2 regulated apoptosis in cancer
Abstract
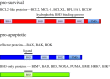
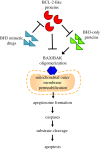
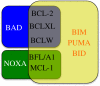
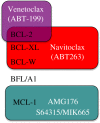
The ability of a cell to undergo mitochondrial apoptosis is governed by pro- and anti-apoptotic members of the BCL-2 protein family. The equilibrium of pro- versus anti-apoptotic BCL-2 proteins ensures appropriate regulation of programmed cell death during development and maintains organismal health. When unbalanced, the BCL-2 family can act as a barrier to apoptosis and facilitate tumour development and resistance to cancer therapy. Here we discuss the BCL-2 family, their deregulation in cancer and recent pharmaceutical developments to target specific members of this family as cancer therapy.
Keywords: BAX/BAK; BCL-2 family; apoptosis.
© 2018 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
-
- Meier P, Finch A, Evan G. 2000. Apoptosis in development. Nature 407, 796–801. (doi:10.1038/35037734) - DOI - PubMed
-
- Oltersdorf T, et al. 2005. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681. (doi:10.1038/nature03579) - DOI - PubMed
-
- Souers AJ, et al. 2013. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208. (doi:10.1038/nm.3048) - DOI - PubMed
-
- Kotschy A, et al. 2016. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538, 477–482. (doi:10.1038/nature19830) - DOI - PubMed
-
- Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. 1984. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226, 1097–1099. (doi:10.1126/science.6093263) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
