Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial
- PMID: 29769450
- PMCID: PMC6012501
- DOI: 10.1172/jci.insight.99145
Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial
Abstract
Background: Heat shock protein peptide complex-96 (HSPPC-96) triggers adaptive and innate antitumor immune responses. The safety and efficacy of HSPPC-96 vaccination was examined in patients with newly diagnosed glioblastoma multiforme (GBM).
Methods: In this open-label, single-arm, phase I study, adult patients were vaccinated with HSPPC-96 in combination with the standard treatment for newly diagnosed GBM after surgical resection. Primary endpoints were frequency of adverse events and progression-free survival (PFS) at 6 months. Secondary endpoints included overall survival (OS), PFS, and tumor-specific immune response (TSIR).
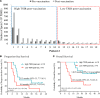
Results: A total of 20 patients with newly diagnosed GBM were enrolled from September 2013 to February 2015. No grade 3 or 4 vaccine-related adverse events were noted. After a median follow-up of 42.3 months, PFS was 89.5% (95% CI, 66.9%-98.7%) at 6 months, median PFS was 11.0 months (95% CI, 8.2-13.8), and median OS was 31.4 months (95% CI, 14.9-47.9). TSIR was significantly increased by 2.3-fold (95% CI, 1.7-3.2) after vaccination. Median OS for patients with high TSIR after vaccination was >40.5 months (95% CI, incalculable) as compared with 14.6 months (95% CI, 7.0-22.2) for patients with low TSIR after vaccination (hazard ratio, 0.25; 95% CI, 0.071-0.90; P = 0.034). A multivariate Cox regression model revealed TSIR after vaccination as a primary independent predicator for survival.
Conclusion: The HSPPC-96 vaccination, combined with the standard therapy, is a safe and effective strategy for treatment of newly diagnosed GBM patients. TSIR after vaccination would be a good indicator predicting the vaccine efficacy.
Trial registration: ClinicalTrials.gov NCT02122822.
Funding: National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2014BAI04B01, 2014BAI04B02), Beijing Natural Science Foundation (7164253), Beijing Talents Fund (2014000021469G257), and Shenzhen Science and Technology Innovation Committee (JSGG20170413151359491).
Keywords: Brain cancer; Clinical Trials; Vaccines.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

