The kinematic recovery process of rhesus monkeys after spinal cord injury
- PMID: 29769463
- PMCID: PMC6219880
- DOI: 10.1538/expanim.18-0023
The kinematic recovery process of rhesus monkeys after spinal cord injury
Abstract
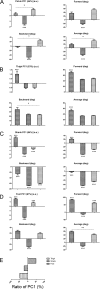
After incomplete spinal cord injury (SCI), neural circuits may be plastically reconstructed to some degree, resulting in extensive functional locomotor recovery. The present study aimed to observe the post-SCI locomotor recovery of rhesus monkey hindlimbs and compare the recovery degrees of different hindlimb parts, thus revealing the recovery process of locomotor function. Four rhesus monkeys were chosen for thoracic hemisection injury. The hindlimb locomotor performance of these animals was recorded before surgery, as well as 6 and 12 weeks post-lesion. Via principal component analysis, the relevant parameters of the limb endpoint, pelvis, hindlimb segments, and joints were processed and analyzed. Twelve weeks after surgery, partial kinematic recovery was observed at the limb endpoint, shank, foot, and knee joints, and the locomotor performance of the ankle joint even recovered to the pre-lesion level; the elevation angle of the thigh and hip joints showed no obvious recovery. Generally, different parts of a monkey hindlimb had different spontaneous recovery processes; specifically, the closer the part was to the distal end, the more extensive was the locomotor function recovery. Therefore, we speculate that locomotor recovery may be attributed to plastic reconstruction of the motor circuits that are mainly composed of corticospinal tract. This would help to further understand the plasticity of motor circuits after spinal cord injury.
Keywords: locomotion; monkey; spinal cord injury; spontaneous recovery.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

