Antimicrobial use and antimicrobial resistance trends in Canada: 2014
- PMID: 29769991
- PMCID: PMC5757741
- DOI: 10.14745/ccdr.v42i11a02
Antimicrobial use and antimicrobial resistance trends in Canada: 2014
Abstract
Background: There is a global concern that the emergence of antimicrobial resistance (AMR) threatens our ability to treat infectious diseases. The Canadian Antimicrobial Resistance Surveillance System (CARSS) was created in response to the Government of Canada's commitment to addressing AMR. CARSS integrates information from nine different national surveillance systems for tracking antimicrobial use (AMU) and AMR in both humans and animals to inform AMU/AMR research and policy.
Objective: To provide highlights of CARSS data on antimicrobial use in humans and animals, AMR trends in human infections in both hospital and community settings and AMR bacteria found in food production animals.
Methods: Information on AMU in animals and humans is purchased and additional information on AMU in animals is collected through the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS). AMR data in humans focuses on first priority organisms. Data on priority organisms for hospital-based AMR is collected through Canadian Nosocomial Infection Surveillance Program (CNISP), Canadian Tuberculosis Laboratory Surveillance System (CTBLSS), Canadian Tuberculosis Laboratory Surveillance System (CTBRS), Canadian Tuberculosis Reporting System (CTBRS) and CIPARS. Data on community-based AMR is collected through CTBLSS, CTBRS, CIPARS, the Antimicrobial-resistant Neisseria gonorrhoeae Surveillance System (ARNGSS) and the National Surveillance of Invasive Streptococcal Disease (NSISD). AMR data on animals is collected through CIPARS.
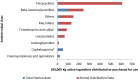
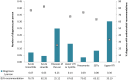
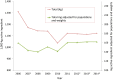
Results: In terms of antibiotic usage in 2014, approximately 82% of antimicrobials were directed to food production animals, 18% to humans and less than one percent each to companion animals (e.g., pets) and crops. Over the past five years, 73% of antimicrobials distributed to food production animals belonged to the same classes as those used in human medicine. Antibiotic usage in humans has remained relatively stable. Trends in 2014 for AMR in hospitals include declining rates of hospital-acquired Clostridium difficile to 3.4 cases per 1,000 patient admissions, methicillin-resistant Staphylococcus aureus (MRSA) infections to 2.89 cases per 10,000 patient days and vancomycin-resistant Enterococci (VRE) to 0.45 cases per 10,000 patient days. Resistance to a number of antimicrobials used to treat Streptococcus pneumoniae has decreased since the introduction of pneumococcal vaccine in 2010. In contrast, trends in 2014 for AMR in the community included increasing rates of community-acquired N. gonorrhoeae - 52.4% of isolates were resistant to at least one antibiotic. Trends for carbapenem-resistant Enterobacteriaceae (CRE) were stable at 0.22 cases per 10,000 patient days. Also, between 2004 and 2014, nine percent of tuberculosis (TB) culture positive cases were resistant to at least one first line anti-tuberculosis drug and this has remained relatively stable over that time. Trends in 2014 for AMR in food production animals showed decreasing resistance of Escherichia coli and Salmonella species to third-generation cephalosporins (ceftriaxone) in poultry associated with a decrease in cephalosporin use on chicken farms, but resistance to ciprofloxacin in Campylobacter species in chicken and cattle has been increasing.
Conclusion: Overall, antibiotic use in humans has not declined despite concerns about overuse. Although resistance rates of C. difficile, VRE, MRSA and AMR S. pneumoniae have been gradually decreasing and drug-resistant tuberculosis and CRE have remained stable, community-associated drug-resistant N. gonorrhoeae has been increasing. Although efforts to decrease antibiotic use in animals have been met with some success, AMR continues to occur in fairly high levels in food production animals.
Conflict of interest statement
Conflict of interest: None.
Figures
References
-
- World Health Organization [Internet]. Antimicrobial resistance: Global report on surveillance 2014. Geneva: WHO; June 2014. Available from: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?....
-
- O’Neill J [Internet]. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. London, UK: Review on Antimicrobial Resistance; December 2014. Available from: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Ta....
-
- Centers for Disease Control and Prevention [Internet]. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS). Antibiotic use in food production animals. Atlanta, GA: CDC; 2014. [updated 2016 Mar 29; cited 2016 Oct 05]. Available from: https://www.cdc.gov/narms/animals.html.
-
- Public Health Agency of Canada [Internet]. Canadian Antimicrobial Resistance Surveillance System: Report 2015. Ottawa, ON: PHAC; March 2015. Available from: http://healthycanadians.gc.ca/alt/pdf/publications/drugs-products-medica....
-
- Public Health Agency of Canada [Internet]. Canadian Antimicrobial Resistance Surveillance System – Report 2016. Ottawa, ON: PHAC; September 2016. Available from: http://healthycanadians.gc.ca/publications/drugs-products-medicaments-pr....
LinkOut - more resources
Full Text Sources
Miscellaneous