Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications
- PMID: 29770269
- PMCID: PMC5951134
- DOI: 10.7717/peerj.4370
Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications
Abstract
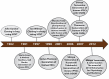
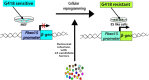
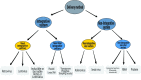
The discovery of induced pluripotent stem cells (iPSCs) by Shinya Yamanaka in 2006 was heralded as a major breakthrough of the decade in stem cell research. The ability to reprogram human somatic cells to a pluripotent embryonic stem cell-like state through the ectopic expression of a combination of embryonic transcription factors was greeted with great excitement by scientists and bioethicists. The reprogramming technology offers the opportunity to generate patient-specific stem cells for modeling human diseases, drug development and screening, and individualized regenerative cell therapy. However, fundamental questions have been raised regarding the molecular mechanism of iPSCs generation, a process still poorly understood by scientists. The efficiency of reprogramming of iPSCs remains low due to the effect of various barriers to reprogramming. There is also the risk of chromosomal instability and oncogenic transformation associated with the use of viral vectors, such as retrovirus and lentivirus, which deliver the reprogramming transcription factors by integration in the host cell genome. These challenges can hinder the therapeutic prospects and promise of iPSCs and their clinical applications. Consequently, extensive studies have been done to elucidate the molecular mechanism of reprogramming and novel strategies have been identified which help to improve the efficiency of reprogramming methods and overcome the safety concerns linked with iPSC generation. Distinct barriers and enhancers of reprogramming have been elucidated, and non-integrating reprogramming methods have been reported. Here, we summarize the progress and the recent advances that have been made over the last 10 years in the iPSC field, with emphasis on the molecular mechanism of reprogramming, strategies to improve the efficiency of reprogramming, characteristics and limitations of iPSCs, and the progress made in the applications of iPSCs in the field of disease modelling, drug discovery and regenerative medicine. Additionally, this study appraises the role of genomic editing technology in the generation of healthy iPSCs.
Keywords: CRISPR; Cell therapy; Embryonic stem cells; Gene editing technology; Induced pluripotent stem cells; Reprogramming; Reprogramming factors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, Edel M, Boue S, Izpisua Belmonte JC. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature Biotechnology. 2008;26(11):1276–1284. doi: 10.1038/nbt.1503. - DOI - PubMed
-
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, Nishikawa S. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(34):14234–14239. doi: 10.1073/pnas.1103509108. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

