Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models
- PMID: 29770526
- PMCID: PMC6158106
- DOI: 10.1002/stem.2852
Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models
Abstract
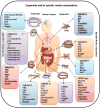
Three-dimensional (3D) cultures use the property of some cells to self-organize in matrices and generate structures that can be programmed to represent an organ or a pathology. Organoid cultures are the 3D cultivation of source tissue (ranging from cells to tissue fragments) in a support matrix and specialized media that nearly resembles the physiological environment. Depending on the source tissue, growth factors, and inhibitors provided, organoids can be programmed to recapitulate the biology of a system and progression of pathology. Organoids are genetically stable, and genetically amenable, making them very suitable tools to study tissue homeostasis and cancer. In this Review, we focus on providing recent technical advances from published literature to efficiently use organoids as a tool for disease modeling and therapeutics. Also, we discuss stem cell biology principles used to generate multiple organoids and their characteristics, with a brief description of methodology. A major theme of this review is to expand organoid applications to the study disease progression and drug response in different cancers. We also discuss shortcomings, limitations, and advantages of developed 3D cultures, with the rationale behind the methodology. Stem Cells 2018;36:1329-1340.
Keywords: Adult stem cells; Differentiation; Experimental models; Induced pluripotent stem cells; Organoids; Stem cell culture; Three-dimensional culture; Tumoroid.
© AlphaMed Press 2018.
Conflict of interest statement
The authors indicated no potential conflict of interest.
Figures


References
-
- Huch M. Building stomach in a dish. Nat Cell Biol. 2015;17:966–967. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

