Impact of increased utilization of neoadjuvant chemotherapy on survival in patients with advanced ovarian cancer: experience from a comprehensive cancer center
- PMID: 29770632
- PMCID: PMC5981113
- DOI: 10.3802/jgo.2018.29.e63
Impact of increased utilization of neoadjuvant chemotherapy on survival in patients with advanced ovarian cancer: experience from a comprehensive cancer center
Abstract
Objective: The choice between primary debulking surgery (PDS) and neoadjuvant chemotherapy (NAC) in advanced ovarian cancer remains controversial. We evaluated NAC use in our center before and after results from a randomized trial were published, with the aim to determine the impact of changes in the neoadjuvant strategy on survival in advanced-stage ovarian cancer.
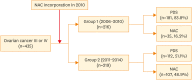
Methods: We retrospectively investigated the clinical course of 435 patients with ovarian, tubal, or peritoneal carcinoma (International Federation of Gynecology and Obstetrics [FIGO] stage III or IV). According to the period of treatment, we stratified patients into a control group (n=216; diagnosed between 2006 and 2010; 83.8% underwent PDS) and a study group (n=219; diagnosed between 2011 and 2014; 48.9% received NAC followed by interval debulking surgery [IDS]).
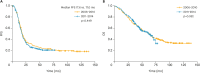
Results: There were no between-group differences in age, body mass index, histology findings, or tumor grade. Compared to patients in the control group, those in the study group were more likely to receive NAC followed by IDS as first-line treatment (48.9% vs. 16.2%; p<0.001), cytoreductive surgery to no-residual disease (21.5% vs. 10.2%; p<0.001), or radical surgery (57.5% vs. 35.6%; p<0.001). However, there was no between-group difference in postoperative morbidity. Kaplan-Meier analysis showed no between-group differences in progression-free or overall survival (p=0.449 and 0.952, respectively).
Conclusion: NAC incorporation resulted in increased optimal cytoreduction rates although no significant differences in survival outcomes were noted. NAC is advantageous for patients with high perioperative morbidity or unresectable disease.
Keywords: Chemotherapy, Adjuvant; Debulking Surgical Procedure; Neoadjuvant Therapy; Ovarian Neoplasms.
Copyright © 2018. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005;50:426–438. - PubMed
-
- Morgan RJ, Jr, Alvarez RD, Armstrong DK, Burger RA, Castells M, Chen LM, et al. Ovarian cancer, version 3.2012. J Natl Compr Canc Netw. 2012;10:1339–1349. - PubMed
-
- Onda T, Matsumoto K, Shibata T, Sato A, Fukuda H, Konishi I, et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol. 2008;38:74–77. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous