Gingival Mesenchymal Stem Cells Outperform Haploidentical Dental Pulp-derived Mesenchymal Stem Cells in Proliferation Rate, Migration Ability, and Angiogenic Potential
- PMID: 29770705
- PMCID: PMC6050910
- DOI: 10.1177/0963689718759649
Gingival Mesenchymal Stem Cells Outperform Haploidentical Dental Pulp-derived Mesenchymal Stem Cells in Proliferation Rate, Migration Ability, and Angiogenic Potential
Abstract
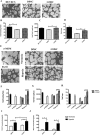
High donor variation makes comparison studies between different dental sources dubious. Dental tissues offer a rare opportunity for comparing the biological characteristics of haploidentical mesenchymal stem cells (MSCs) isolated from the same donor. The objective was to identify the optimal dental source of MSCs through a biological and functional comparison of haploidentical MSCs from gingival (GMSCs) and dental pulp stem cells (DPSCs) focusing mainly on their angiogenic potential. The comparison study included (1) surface markers expression, (2) mesodermal differentiation capacity (chondrogenic, adipogenic, and osteogenic), (3) proliferation, (4) migration potential, (5) ability to form colony units, and (6) angiogenic potential in vitro and in vivo. Comparative analysis showed no difference in the immunophenotypic profile nor for the trilineage differentiation potential. Proliferation of GMSCs was higher than DPSCs at day 6 (2.6-fold higher, P < 0.05). GMSCs showed superior migratory capacity compared to DPSCs at 4, 8, and 12 h (2.1-, 1.5-, and 1.2-fold higher, respectively, P < 0.05). Furthermore, GMSCs formed a higher number of colony units for both cell concentrations (1.7- and 1.4-fold higher for 150 and 250 starting cells, respectively, P < 0.05). GMSCs showed an improved angiogenic capacity compared to DPSCs (total tube lengths 1.17-fold higher and 1.5-fold total loops, P < 0.05). This was correlated with an enhanced release of vascular growth factor under hypoxic conditions. Finally, in the plug transplantation assay evaluating the angiogenesis in vivo, the DPSC and GMSC hemoglobin content was 3.9- and 4-fold higher, respectively, when compared to the control (Matrigel alone). GMSCs were superior to their haploidentical DPSCs in proliferation, migration, and angiogenic potentials. This study positions GMSCs in the forefront of dental cell sources for applications in regenerative medicine.
Keywords: angiogenesis; dental and gingival mesenchymal stem cells; haploidentical; migration.
Conflict of interest statement
Figures




References
-
- Chen FM, Sun HH, Lu H, Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012;33(27):6320–6344. - PubMed
-
- Yang Y, Rossi FM, Putnins EE. Periodontal regeneration using engineered bone marrow mesenchymal stromal cells. Biomaterials. 2010;31(33):8574–8582. - PubMed
-
- Tobita M, Uysal AC, Ogawa R, Hyakusoku H, Mizuno H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A. 2008;14(6):945–953. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

