Direct-acting antiviral agents in patients with hepatitis C genotype 1-4 infections in a tertiary hospital
- PMID: 29771105
- PMCID: PMC6166256
Direct-acting antiviral agents in patients with hepatitis C genotype 1-4 infections in a tertiary hospital
Abstract
Objective: Hepatitis C virus (HCV) infection is a major cause of chronic liver disease. Six different genotypes (GT) of HCV (genotypes 1-6) have been identified. The genotype is clinically relevant since the majority of current direct antiviral agents (DAA´s) do not have pangenotypic efficacy. The purpose of this study was to describe the clinical characteristics of real world patients and evaluate the effectiveness of different treatment regimens.
Methods: Retrospective and observational study carried out in a third level hospital. Study period: January 2015-January 2016. Inclusion criteria: HCV patients of any genotype treated with either DAAs ± rivabirin (RBV) or DAAs + RBV + pegilated interferon (Peg-IFN) regimens for 12 weeks. Exclusion criteria: patients without adequate clinical or analytical information available for further analysis. Patients treated for 24 weeks were excluded. The main endpoint was sustained virologic response 12 weeks after the end of treatment (SVR12), and secondary endpoint was SVR24.
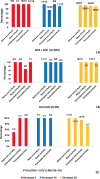
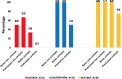
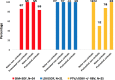
Results: A total of 515 patients were included (aged 55.52±8.97 years). GT1: patients treated with simeprevir + sofosbuvir (SIM + SOF), ledipavir (LDV)/SOF and paritaprevir/ritonavir/ombitasvir + dasabuvir (PTV/r/OBV + DSV) ± RBV had a SVR12 of 93.59% (190/203), 98.82% (N=84/85), 94.28% (66/70), respectively. Regarding daclatasvir (DCV) + SOF and SIM + DCV, everybody (19/19) and 87.5% (7/8) got SVR12, respectively. GT2: 71.42% (N=10/14) of patients achieved SVR12, concretely, SOF + RBV had a SVR12 75% (N=6/8). GT3: 43.75% (N=7/16), 90% (N=9/10) and 95% (N=19/20) of patients treated with LDV/SOF, LDV/SOF + RBV and SOF + DCV obtained SVR12, respectively. GT4: patients treated with LDV/SOF, SIM + SOF and PTV/r/OBV ± RBV had a SVR12 rate of 100% (21/21), 91.67% (22/24) and 92% (23/25), respectively. All patients that got SVR12 achieved SVR24.
Conclusions: Our study confirmed the efficacy data reported in clinical trials in a cohort of patients with GT1-4 and a wide range of basal characteristics.
Introducción: La infección por el virus de la hepatitis C (VHC) es una causa importante de enfermedad hepática crónica. Se han identificado seis genotipos (GT) diferentes de VHC (genotipos 1-6). El genotipo es relevante dado que la mayoría de los antivirales de acción directa (AAD) actuales no tienen eficacia pangenotípica. El objetivo del presente estudio fue describir las características clínicas de los pacientes y evaluar la efectividad de los diferentes tratamientos en condiciones de uso real.
Material y métodos: Estudio observacional, retrospectivo realizado en un hospital de tercer nivel. Período de estudio: enero-2015 a enero-2016. Criterios de inclusión: pacientes con VHC de cualquier genotipo tratados con AAD ± ribavirina (RBV) o AAD + RBV + interferón-α pegilado (Peg-IFN) durante 12 semanas. Criterios de exclusión: pacientes de quienes no se dispuso de información clínica/analítica adecuada para análisis posterior. Los pacientes tratados durante 24 semanas fueron excluidos. La variable principal fue la respuesta viral sostenida 12 semanas después de terminar el tratamiento (RVS12) y la secundaria la RVS24.
Resultados: Se incluyeron 515 pacientes (55,52 ± 8,97 años). GT1: pacientes tratados con simeprevir + sofosbuvir (SIM + SOF), ledipavir (LDV)/SOF y paritaprevir/ritonavir/ombitasvir + dasabuvir (PTV/r/OBV + DSV) ± RBV, tuvieron una RVS12 de 93,59% (190/203), 98,82% (84/85), 94,28% (66/70). En cuanto a daclatasvir (DCV) + SOF y SIM + DCV, todos (19/19) y 87,5% (7/8) obtuvieron RVS12, respectivamente. GT2: 71,42% (10/14) de los pacientes lograron RVS12, concretamente, los tratados con SOF+RBV tuvieron una RVS12 75% (6/8). GT3: 43,75% (7/16), 90% (9/10) y 95% (19/20) de los pacientes tratados con LDV/SOF, LDV/SOF + RBV y SOF + DCV alcanzaron RVS12, correspondientemente. GT4: pacientes tratados con LDV/SOF, SIM + SOF y PTV/r/OBV ± RBV tuvieron RVS12 del 100% (21/21), 91,67% (22/24) y 92% (23/25), respectivamente. Todos los pacientes que obtuvieron RVS12 lograron RVS24.
Conclusión: Nuestro estudio confirmó los datos de eficacia publicados en los ensayos clínicos en una cohorte de pacientes con GT1-4 y con una amplia gama de características basales.
©The Author 2018. Published by Sociedad Española de Quimioterapia. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)(https://creativecommons.org/licenses/by-nc/4.0/).
Conflict of interest statement
The authors declare that they have no conflicts of interest
Figures

Similar articles
-
Effectiveness of 12 week ledipasvir/sofosbuvir and predictors of treatment failure in patients with hepatitis C.Rev Esp Quimioter. 2019 Aug;32(4):296-302. Epub 2019 Jun 21. Rev Esp Quimioter. 2019. PMID: 31232572 Free PMC article.
-
Interferon-free treatments in patients with hepatitis C genotype 3 infection in a tertiary hospital.Rev Esp Quimioter. 2018 Feb;31(1):35-42. Epub 2018 Jan 29. Rev Esp Quimioter. 2018. PMID: 29376623 Free PMC article.
-
Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort.J Hepatol. 2017 Jun;66(6):1138-1148. doi: 10.1016/j.jhep.2017.01.028. Epub 2017 Feb 9. J Hepatol. 2017. PMID: 28189751
-
Hepatitis C: efficacy and safety in real life.Liver Int. 2017 Jan;37 Suppl 1:26-32. doi: 10.1111/liv.13293. Liver Int. 2017. PMID: 28052633 Review.
-
Direct-acting antiviral agents for liver transplant recipients with recurrent genotype 1 hepatitis C virus infection: Systematic review and meta-analysis.Transpl Infect Dis. 2019 Apr;21(2):e13047. doi: 10.1111/tid.13047. Epub 2019 Jan 21. Transpl Infect Dis. 2019. PMID: 30615227 Free PMC article.
Cited by
-
Effectiveness of 12 week ledipasvir/sofosbuvir and predictors of treatment failure in patients with hepatitis C.Rev Esp Quimioter. 2019 Aug;32(4):296-302. Epub 2019 Jun 21. Rev Esp Quimioter. 2019. PMID: 31232572 Free PMC article.
-
Consensus on management of hepatitis C virus infection in resource-limited Ukraine and Commonwealth of Independent States regions.World J Gastroenterol. 2019 Aug 7;25(29):3897-3919. doi: 10.3748/wjg.v25.i29.3897. World J Gastroenterol. 2019. PMID: 31413526 Free PMC article. Review.
-
Sustained virological response in patients with HCV treated with daclatasvir plus sofosbuvir, with or without ribavirin: a large, field-practice study.Drugs Context. 2020 Dec 15;9:2020-4-11. doi: 10.7573/dic.2020-4-11. eCollection 2020. Drugs Context. 2020. PMID: 33408749 Free PMC article.
-
Efficacy and Safety of Generic Sofosbuvir Plus Daclatasvir and Sofosbuvir/Velpatasvir in HCV Genotype 3-Infected Patients: Real-World Outcomes From Pakistan.Front Pharmacol. 2020 Sep 2;11:550205. doi: 10.3389/fphar.2020.550205. eCollection 2020. Front Pharmacol. 2020. PMID: 32982753 Free PMC article.
References
-
- Centro de información online de medicamentos de la AEMPS [Internet].. Madrid: Agencia Española del Medicamento y Productos Sanitarios; 1997- [Cited 2018 January 07]. Available from: https://www.aemps.gob.es/cima/publico/home.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

