Paced breathing and phrenic nerve responses evoked by epidural stimulation following complete high cervical spinal cord injury in rats
- PMID: 29771608
- PMCID: PMC6734078
- DOI: 10.1152/japplphysiol.00895.2017
Paced breathing and phrenic nerve responses evoked by epidural stimulation following complete high cervical spinal cord injury in rats
Abstract
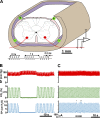
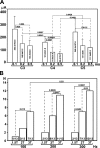
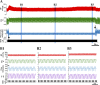
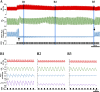
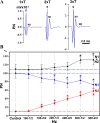
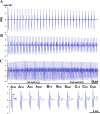
Spinal cord injury (SCI) at the level of cervical segments often results in life-threatening respiratory complications and requires long-term mechanical ventilator assistance. Thus restoring diaphragm activity and regaining voluntary control of breathing are the primary clinical goals for patients with respiratory dysfunction following cervical SCI. Epidural stimulation (EDS) is a promising strategy that has been explored extensively for nonrespiratory functions and to a limited extent within the respiratory system. The goal of the present study is to assess the potential for EDS at the location of the phrenic nucleus (C3-C5) innervating the diaphragm: the main inspiratory muscle following complete C1 cervical transection. To avoid the suppressive effect of anesthesia, all experiments were performed in decerebrate, C1 cervical transection, unanesthetized, nonparalyzed ( n = 13) and paralyzed ( n = 7) animals. Our results show that C4 segment was the most responsive to EDS and required the lowest threshold of current intensity, affecting tracheal pressure and phrenic nerve responses. High-frequency (200-300 Hz) EDS applied over C4 segment (C4-EDS) was able to maintain breathing with normal end-tidal CO2 level and raise blood pressure. In addition, 100-300 Hz of C4-EDS showed time- and frequency-dependent changes (short-term facilitation) of evoked phrenic nerve responses that may serve as a target mechanism for pacing of phrenic motor circuits. The present work provides the first report of successful EDS at the level of phrenic nucleus in a complete SCI animal model and offers insight into the potential therapeutic application in patients with high cervical SCI. NEW & NOTEWORTHY The present work offers the first demonstration of successful life-supporting breathing paced by epidural stimulation (EDS) at the level of the phrenic nucleus, following a complete spinal cord injury in unanesthetized, decerebrate rats. Moreover, our experiments showed time- and frequency-dependent changes of evoked phrenic nerve activity during EDS that may serve as a target mechanism for pacing spinal phrenic motor networks.
Keywords: breathing; complete spinal cord injury; epidural stimulation; phrenic nerve; rats.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Agostoni E, Mognoni P, Torri G, Saracino F. Relation between changes of the rib cage circumference and lung volume. J Appl Physiol 20: 1179–1186, 1965. doi: 10.1152/jappl.1965.20.6.1179. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

