Tracheal acid or surfactant instillation raises alveolar surface tension
- PMID: 29771610
- PMCID: PMC6295485
- DOI: 10.1152/japplphysiol.00397.2017
Tracheal acid or surfactant instillation raises alveolar surface tension
Abstract
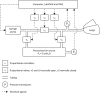
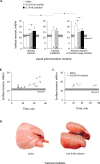
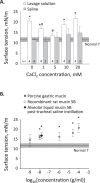
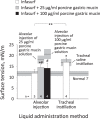
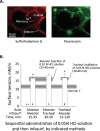
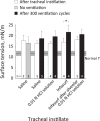
Whether alveolar liquid surface tension, T, is elevated in the acute respiratory distress syndrome (ARDS) has not been demonstrated in situ in the lungs. Neither is it known how exogenous surfactant, which has failed to treat ARDS, affects in situ T. We aim to determine T in an acid-aspiration ARDS model before and after exogenous surfactant administration. In isolated rat lungs, we combine servo-nulling pressure measurement and confocal microscopy to determine alveolar liquid T according to the Laplace relation. Administering 0.01 N (pH 1.9) HCl solution by alveolar injection or tracheal instillation, to model gastric liquid aspiration, raises T. Subsequent surfactant administration fails to normalize T. Furthermore, in normal lungs, tracheal instillation of control saline or exogenous surfactant raises T. Lavaging the trachea with saline and injecting the lavage solution into the alveolus raises T, suggesting that tracheal instillation may wash T-raising airway contents to the alveolus. Adding 0.01 N HCl or 5 mM CaCl2-either of which aggregates mucins-to tracheal lavage solution reduces or eliminates the effect of lavage solution on alveolar T. Following tracheal saline instillation, liquid suctioned directly out of alveoli through a micropipette contains mucins. Additionally, alveolar injection of gastric mucin solution raises T. We conclude that 1) tracheal liquid instillation likely washes T-raising mucins to the alveolus and 2) even exogenous surfactant that could be delivered mucin-free to the alveolus might not normalize T in acid-aspiration ARDS. NEW & NOTEWORTHY We demonstrate in situ in isolated lungs that surface tension is elevated in an acid-aspiration acute respiratory distress syndrome (ARDS) model. Following tracheal liquid instillation, also in isolated lungs, we directly sample alveolar liquid. We find that liquid instillation into normal lungs washes mucins to the alveolus, thereby raising alveolar surface tension. Furthermore, even if exogenous surfactant could be delivered mucin-free to the alveolus, exogenous surfactant might fail to normalize alveolar surface tension in acid-aspiration ARDS.
Keywords: acid aspiration; acute respiratory distress syndrome; airway mucins; exogenous surfactant therapy; tracheal instillation.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

