Septins regulate junctional integrity of endothelial monolayers
- PMID: 29771630
- PMCID: PMC6080707
- DOI: 10.1091/mbc.E18-02-0136
Septins regulate junctional integrity of endothelial monolayers
Abstract
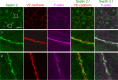
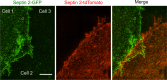
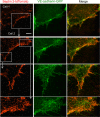
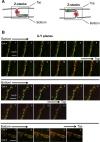
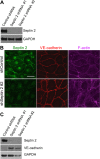
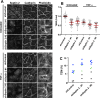
Junctional integrity of endothelial monolayers is crucial to control movement of molecules and cells across the endothelium. Examining the structure and dynamics of cell junctions in endothelial monolayers, we discovered a role for septins. Contacts between adjacent endothelial cells were dynamic, with protrusions extending above or below neighboring cells. Vascular endothelial cadherin (VE-cadherin) was present at cell junctions, with a membrane-associated layer of F-actin. Septins localized at cell-junction membranes, in patterns distinct from VE-cadherin and F-actin. Septins assumed curved and scallop-shaped patterns at junctions, especially in regions of positive membrane curvature associated with actin-rich membrane protrusions. Depletion of septins led to disrupted morphology of VE-cadherin junctions and increased expression of VE-cadherin. In videos, septin-depleted cells displayed remodeling at cell junctions; regions with VE-cadherin were broader, and areas with membrane ruffling were wider. Septin depletion and junction disruption led to functional loss of junctional integrity, revealed by decreased transendothelial electric resistance and increased transmigration of immune cells. We conclude that septins, as cytoskeletal elements associated with the plasma membrane, are important for cell junctions and junctional integrity of endothelial monolayers, functioning at regions of positive curvature in support of actin-rich protrusions to promote cadherin-based cell junctions.
Figures



References
-
- Abbott NJ, Rönnbäck L, Hansson E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci , 41–53. - PubMed
-
- Baldwin AL, Thurston G. (2001). Mechanics of endothelial cell architecture and vascular permeability. Crit Rev Biomed Eng , 247–278. - PubMed
-
- Bazzoni G, Dejana E. (2004). Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev , 869–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

