Vitamin E Phosphate Coating Stimulates Bone Deposition in Implant-related Infections in a Rat Model
- PMID: 29771856
- PMCID: PMC6125746
- DOI: 10.1097/01.blo.0000534692.41467.02
Vitamin E Phosphate Coating Stimulates Bone Deposition in Implant-related Infections in a Rat Model
Abstract
Background: Implant-related infections are associated with impaired bone healing and osseointegration. In vitro antiadhesive and antibacterial properties and in vivo antiinflammatory effects protecting against bone loss of various formulations of vitamin E have been demonstrated in animal models. However, to the best of our knowledge, no in vivo studies have demonstrated the synergistic activity of vitamin E in preventing bacterial adhesion to orthopaedic implants, thus supporting the bone-implant integration.
Questions/purposes: The purpose of this study was to test whether a vitamin E phosphate coating on titanium implants may be able to reduce (1) the bacterial colonization of prosthetic implants and (2) bone resorption and osteomyelitis in a rat model of Staphylococcus aureus-induced implant-related infection.
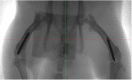
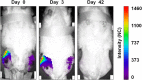
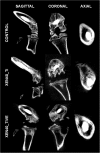
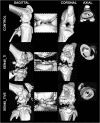
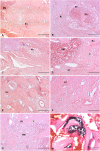
Methods: Twelve rats were bilaterally injected in the femurs with S aureus UAMS-1-Xen40 and implanted with uncoated or vitamin E phosphate-coated titanium Kirschner wires without local or systemic antibiotic prophylaxis. Eight rats represented the uninfected control group. A few hours after surgery, two control and three infected animals died as a result of unexpected complications. With the remaining rats, we assessed the presence of bacterial contamination with qualitative bioluminescence imaging and Gram-positive staining and with quantitative bacterial count. Bone changes in terms of resorption and osteomyelitis were quantitatively analyzed through micro-CT (bone mineral density) and semiquantitatively through histologic scoring systems.
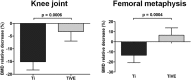
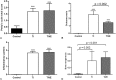
Results: Six weeks after implantation, we found only a mild decrease in bacterial count in coated versus uncoated implants (Ti versus controls: mean difference [MD], -3.705; 95% confidence interval [CI], -4.416 to -2.994; p < 0.001; TiVE versus controls: MD, -3.063; 95% CI, -3.672 to -2.454; p < 0.001), whereas micro-CT analysis showed a higher bone mineral density at the knee and femoral metaphysis in the vitamin E-treated group compared with uncoated implants (knee joint: MD, -11.88; 95% CI, -16.100 to -7.664; p < 0.001 and femoral metaphysis: MD, -19.87; 95% CI, -28.82 to -10.93; p < 0.001). We found decreased osteonecrosis (difference between medians, 1.5; 95% CI, 1-2; p < 0.002) in the infected group receiving the vitamin E-coated nails compared with the uncoated nails.
Conclusions: These preliminary findings indicate that vitamin E phosphate implant coatings can exert a protective effect on bone deposition in a highly contaminated animal model of implant-related infection.
Clinical relevance: The use of vitamin E coatings may open new perspectives for developing coatings that can limit septic loosening of infected implants with bacterial contamination. However, a deeper insight into the mechanism of action and the local release of vitamin E as a coating for orthopaedic implants is required to be used in clinics in the near future. Although this study cannot support the antimicrobial properties of vitamin E, promising results were obtained for bone-implant osseointegration. These preliminary results will require further in vivo investigations to optimize the host response in the presence of antibiotic prophylaxis.
Conflict of interest statement
All ICMJE Conflict of Interest Forms for authors and
Figures
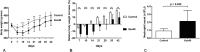

Comment in
-
CORR Insights®: Vitamin E Phosphate Coating Stimulates Bone Deposition in Implant-related Infections in a Rat Model.Clin Orthop Relat Res. 2018 Jun;476(6):1339-1340. doi: 10.1007/s11999.0000000000000304. Clin Orthop Relat Res. 2018. PMID: 29629938 Free PMC article. No abstract available.
References
-
- Aberg J, Henriksson HB, Engqvist H, Palmquist A, Brantsing C, Lindahl A, Thomsen P, Brisby H. Biocompatibility and resorption of a radiopaque premixed calcium phosphate cement. J Biomed Mater Res A. 2001;100:1269–1278. - PubMed
-
- Al-Salih DAAK, Aziz FM, Mshimesh BAR, Jehad MT. Antibacterial effects of vitamin E: in vitro study. J Biotech Res Center. 2013;7:17–23.
-
- Banche G, Bracco P, Bistolfi A, Allizond V, Boffano M, Costa L, Cimino A, Cuffini AM, Del Prever EM. Vitamin E blended UHMWPE may have the potential to reduce bacterial adhesive ability. J Orthop Res. 2011;29:1662–1667. - PubMed
-
- Bichara DA, Malchau E, Sillesen NH, Cakmak S, Nielsen GP, Muratoglu OK. Vitamin E-diffused highly cross-linked UHMWPE particles induce less osteolysis compared to highly cross-linked virgin UHMWPE particles in vivo. J Arthroplasty. 2014;29:232–237. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

