Another cat and mouse game: Deciphering the evolution of the SCGB superfamily and exploring the molecular similarity of major cat allergen Fel d 1 and mouse ABP using computational approaches
- PMID: 29771985
- PMCID: PMC5957422
- DOI: 10.1371/journal.pone.0197618
Another cat and mouse game: Deciphering the evolution of the SCGB superfamily and exploring the molecular similarity of major cat allergen Fel d 1 and mouse ABP using computational approaches
Abstract
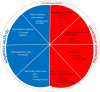
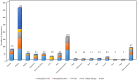
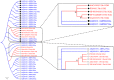
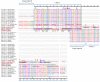
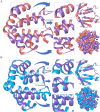
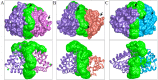
The mammalian secretoglobin (SCGB) superfamily contains functionally diverse members, among which the major cat allergen Fel d 1 and mouse salivary androgen-binding protein (ABP) display similar subunits. We searched for molecular similarities between Fel d 1 and ABP to examine the possibility that they play similar roles. We aimed to i) cluster the evolutionary relationships of the SCGB superfamily; ii) identify divergence patterns, structural overlap, and protein-protein docking between Fel d 1 and ABP dimers; and iii) explore the residual interaction between ABP dimers and steroid binding in chemical communication using computational approaches. We also report that the evolutionary tree of the SCGB superfamily comprises seven unique palm-like clusters, showing the evolutionary pattern and divergence time tree of Fel d 1 with 28 ABP paralogs. Three ABP subunits (A27, BG27, and BG26) share phylogenetic relationships with Fel d 1 chains. The Fel d 1 and ABP subunits show similarities in terms of sequence conservation, identical motifs and binding site clefts. Topologically equivalent positions were visualized through superimposition of ABP A27:BG27 (AB) and ABP A27:BG26 (AG) dimers on a heterodimeric Fel d 1 model. In docking, Fel d 1-ABP dimers exhibit the maximum surface binding ability of AG compared with that of AB dimers and the several polar interactions between ABP dimers with steroids. Hence, cat Fel d 1 is an ABP-like molecule in which monomeric chains 1 and 2 are the equivalent of the ABPA and ABPBG monomers, respectively. These findings suggest that the biological and molecular function of Fel d 1 is similar to that of ABP in chemical communication, possibly via pheromone and/or steroid binding.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Mukherjee AB, Chilton BS. The uteroglobin/Clara cell protein family. Ann N Y Acad Sci. 2000;923: 1–356.
-
- Daniel JC. Discovery and perspectives from the blastokinin era. Ann N Y Acad Sci. 2000;923: 1–8. doi: 10.1111/j.1749-6632.2000.tb05515.x . - DOI - PubMed
-
- Beier HM. The discovery of uteroglobin and its significance for reproductive biology and endocrinology. Ann N Y Acad Sci. 2000;923: 9–24. doi: 10.1111/j.1749-6632.2000.tb05516.x . - DOI - PubMed
-
- Niimi T, Keck-Waggoner CL, Popescu NC, Zhou Y, Levitt RC, Kimura S. UGRP1, a uteroglobin/Clara cell secretory protein-related protein, is a novel lung-enriched downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor. Mol Endocrinol. 2001;15: 2021–2036. doi: 10.1210/mend.15.11.0728 . - DOI - PubMed
-
- Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56: 860–865. . - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

