The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration-A cost-effectiveness analysis from a societal perspective
- PMID: 29772018
- PMCID: PMC5957378
- DOI: 10.1371/journal.pone.0197670
The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration-A cost-effectiveness analysis from a societal perspective
Abstract
Background: The discussion on the use of bevacizumab is still ongoing and often doctors are deterred from using bevacizumab due to legal or political issues. Bevacizumab is an effective, safe and inexpensive treatment option for neovascular age-related macular degeneration (AMD), albeit unregistered for the disease. Therefore, in some countries ophthalmologists use the equally effective but expensive drugs ranibizumab and aflibercept. We describe the economic consequences of this dilemma surrounding AMD treatment from a societal perspective.
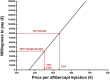
Methods: We modelled cost-effectiveness of treatment with ranibizumab (as-needed), aflibercept (bimonthly) and bevacizumab (as-needed). Effectiveness was estimated by systematic review and meta-analysis. The drug with the most favourable cost-effectiveness profile compared to bevacizumab was used for threshold analyses. First, we determined how much we overspend per injection. Second, we calculated the required effectiveness to justify the current price and the reasonable price for a drug leading to optimal vision. Finally, we estimated how much Europe overspends if bevacizumab is not first choice.
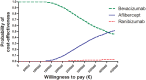
Results: Bevacizumab treatment costs €27,087 per year, about €4,000 less than aflibercept and €6,000 less than ranibizumab. With similar effectiveness for all drugs as shown by meta-analysis, bevacizumab was the most cost-effective. Aflibercept was chosen for threshold analyses. Aflibercept costs €943 per injection, but we determined that the maximum price to be cost-effective is €533. Alternatively, at its current price, aflibercept should yield about twice the visual gain. Even when optimal vision can be achieved, the maximum price for any treatment is €37,453 per year. Most importantly, Europe overspends €335 million yearly on AMD treatment when choosing aflibercept over bevacizumab.
Conclusion: Bevacizumab is the most cost-effective treatment for AMD, yet is not the standard of care across Europe. The registered drugs ranibizumab and aflibercept lead to large overspending without additional health benefits. Health authorities should consider taking steps to implement bevacizumab into clinical practice as first choice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Cohen D. Why have UK doctors been deterred from prescribing Avastin? BMJ. 2015;350:h1654 Epub 2015/04/04. doi: 10.1136/bmj.h1654 . - DOI - PubMed
-
- National Institute for Health Care Excellence (NICE). Ranibizumab and pegaptanib for the treatment of age-related macular degeneration, 2008.
-
- National Institute for Health Care Excellence (NICE). Aflibercept solution for injection for treating wet age-related macular degeneration, 2013.
-
- Office of Inspector General. Medicare Payments for Drugs Used to Treat Wet Age-Related Macular Degeneration. OEI-03-10-00360. Department of Health and Human Services, Washington DC, 2012.
-
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–16. doi: 10.1016/S2214-109X(13)70145-1 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

